Chapter 63 Longitudinal Monitoring of Immune System Parameters of Cetaceans and Application to Their Health Management
Tools for Flow Cytometry–Based Leukocyte Phenotyping
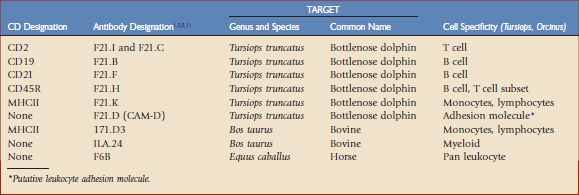
Toothed whales (suborder Odontoceti, order Cetacea), which are the primary focus of this chapter, have no close relatives in the world of domestic mammals, and thus limited immunologic reagents are available to assess perturbations in leukocyte phenotype. Given their monetary value, high visibility, and ongoing training programs directed at establishing voluntary fluke presentation for blood collection, efforts were successfully initiated to develop monoclonal antibodies specific for dolphin (Tursiops truncatus) and killer whale (Orcinus orca) leukocyte differentiation antigens,1–3 referred to in the literature as CD antigens; select monoclonal antibodies previously developed for equine8 and bovine5,11 species were also tested for cross-reactivity. A summary of these antibodies and their leukocyte differentiation antigen specificities, if known, are given in Table 63-1.
Markers
For T and B Lymphocytes
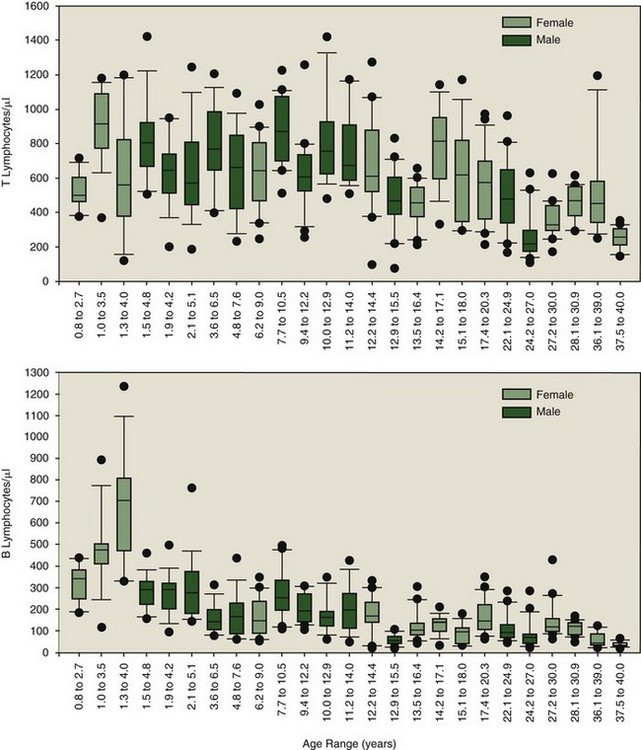
The antibodies shown in Table 63-1 have been applied to four killer whale populations over a period of 12 years. The antibody set was able to distinguish neutrophils (F6B+/ILA-24+, major histocompatibility complex [MHC] II−), monocytes (F6B+/ILA-24+/MHC II+), and lymphocytes (F6B+, MHC II+/ILA-24−). Lymphocytes could be divided into B cells (CD19+, CD21+) and T cells (CD2+), with T cells being subdivided further by differential density expression of CD2 and CD45R into naïve and memory T cell populations (CD2+/CD45R+/Hi and CD2+/CD45R+/Lo, respectively). The usefulness of using a longitudinal approach for identifying immunologic perturbations in killer whales was quickly revealed. As expected, all markers listed in Table 63-1 were determined to be susceptible to perturbation. Outliers could be identified through comparison to the baseline established for the total population. Sensitivity in identification of immunologic perturbations was increased for select animals—low variation in immune parameter values over time—when using their own individual baselines. Absolute numbers of T and B lymphocyte subpopulations, representing a subset of the data collected over a 3-year period from 25 animals, are illustrated in Figure 63-1. Such data may only be developed for the individual through longitudinal sampling. Figure 63-1 illustrates the obvious variability in T and B lymphocyte subpopulations among animals. Those animals with dramatic fluctuations may be experiencing multiple and/or recurring insults, some outwardly visible and others subclinical. On inspection of all leukocyte subpopulation data, total numbers of memory T, naïve T, and B lymphocyte numbers were sometimes abnormally elevated and sometimes depressed; this often occurred differentially, resulting in altered ratios of total T versus B lymphocytes and naïve versus memory T lymphocytes. The clinical significance of the various patterns is currently a matter of investigation. Diagnostically speaking, point in time perturbations and trends toward becoming abnormal may be more readily identified with comparative analyses using a combination of the species’ baseline and an animal’s own individual baseline. However, age-associated changes in leukocyte subpopulation numbers must be taken into account when establishing baseline values for a species.
For Neutrophils
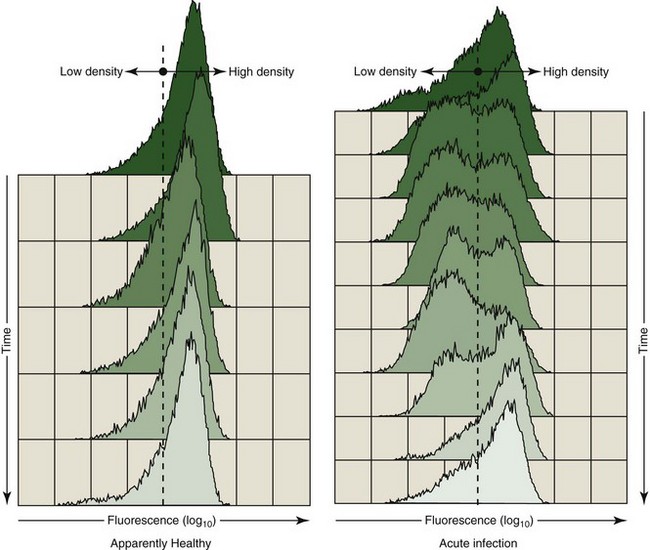
As noted, the cell surface density of select immune parameter markers has provided a sensitive measure of immunologic perturbation. From a diagnostic perspective, the relative density of the putative cell adhesion molecule, CAM-D, appeared to be telling. As determined by flow cytometry, decreased fluorescence intensity on a variable percentage of polymorphonuclear leukocytes (PMNs) stained with fluorescein isothiocyanate (FITC)-labeled α–CAM-D was occasionally recorded. We have speculated that this could be the result of either a conformational change in the adhesion protein, resulting in reduced binding of the monoclonal antibody, or reduced cell surface density of the protein. Regardless, the appearance of a subpopulation of PMNs with low-density fluorescence was associated with clinical disease. Figure 63-2 illustrates a clinically ill animal with a subpopulation of PMNs expressing a low fluorescence as compared with those expressing a relatively high fluorescence following staining with α–CAM-D. Parallel samples obtained from a healthy companion are illustrated for comparative purposes. The first time point was obtained at the initiation of acute illness (top of the figure); the PMN profile slowly returned to a pattern similar to that of the control following treatment.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree