, Monika Hassel2 and Maura Grealy3
(1)
Centre of Organismal Studies, University of Heidelberg, Heidelberg, Germany
(2)
Spezielle Zoologie, Universität Marburg FB Biologie, Marburg, Germany
(3)
Pharmacology and Therapeutics, National University of Ireland Galway, Galway, Ireland
In the development of animals extensive cell migrations take place. This applies especially to vertebrate embryos. They are like cities full of tourists. In a translucent fish embryo migratory cells can be seen crawling and swarming. In avian and mammalian blastodiscs the cells of the meso‘ derm’ do not form a ‘skin’ (Greek derma = skin) or a coherent germ ‘layer’; instead the mesodermal cells creep around like amoebae to colonize the spaces between the ectoderm/epiblast and the endoderm/hypoblast towards their target positions and form a loose primary mesenchyme (see Fig. 5.22 fish, Fig. 5.25 bird). Only later they aggregate forming compact structures such as the somites. When the somites split up, the cells of the sclerotome and dermatome emigrate to form compact structures or soft tissues elsewhere (Fig. 15.1). Primordial germ cells, blood cells and neural crest cells travel particularly long distances. Even the germ cells and the cells of the peripheral nervous system as well as the musculature and the skeletal elements of the extremities (fingers, toes) derive from migratory precursors.
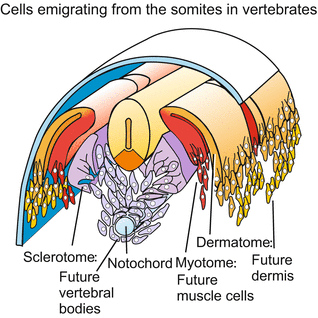

Fig. 15.1
Vertebrate embryo subsequent to neurulation. Fate of cells emigrated from the somites
15.1 Primordial Germ Cells and Blood Cells
15.1.1 Example Primordial Germ Cells Often Migrate Long Distances to Arrive at the Gonads
By definition primordial germ cells are the stem cells of the germ cells. As a rule, primordial germ cells are not generated in the gonads (testis, ovary), but in another place und then migrate into the gonads.
In the colonial hydrozoan Hydractinia, for example, oocytes or spermatocytes originate from pluripotent interstitial stem cells elsewhere in the colony and immigrate into the gonads (gonophores) of sexual polyps (Figs. 4.18 and 18.2). If a male and a female colony grow together male stem cells prowling around can enter the female colony and displace the female germ cells. This, however, occurs only rarely because genetically based histoincompatibility prevents fusion of genetically non-identical (heterogeneic) colonies and thus the invasion of parasitic primordial germ cells. Intruding foreign germ cell precursors would not transmit the genome of the host but their own foreign genome. Genetically fixed histoincompatibility is known also from other sessile colonial animals, for example from the colonial tunicate Botryllus schlosserei. A hypothesis has been proposed assuming histoincompatibility had arisen in evolution to prevent intrusion of parasitic germ cell precursors.
In Drosophila the stem cells of the future germ cells are the first cells to be produced at all. There is no trace of a gonad present at this early stage. The stem cells, called pole cells, are located at the posterior pole of the egg. These pole cells receive so called pole plasm or germ plasm rich with RNA-containing granules and enclosing germ line determinants. These are the products of the genes oskar, vasa and nanos or factors dependent on these genes. During gastrulation, the pole cells are drawn into the interior of the embryo with the invaginating hindgut (Fig. 15.2a). Later, they actively leave the gut and immigrate into the developing ovarioles.
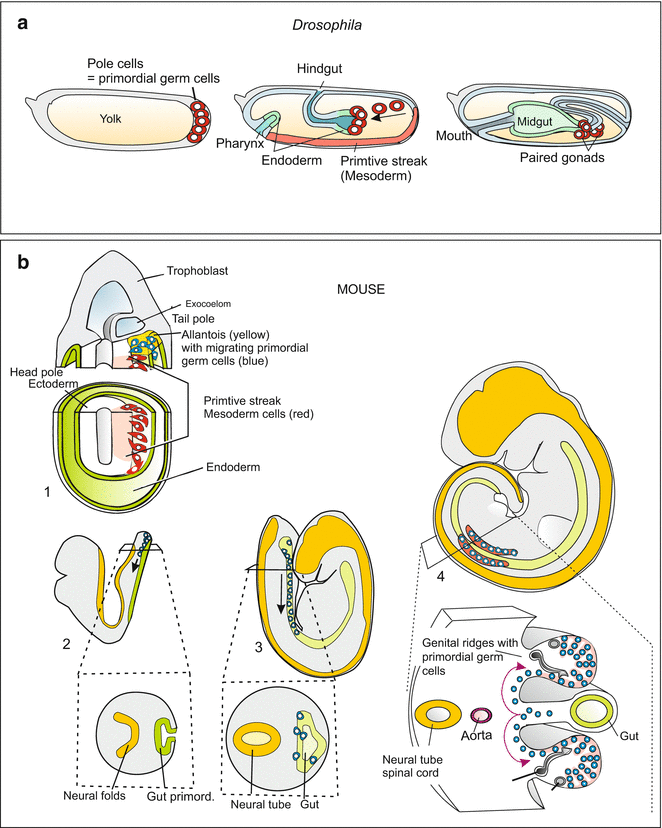

Fig. 15.2
Migrating primordial germ cells. (a) Embryo of Drosophila, (b) Mouse embryo. After Richardson and Lehmann (2010), and other sources
In Xenopus eggs a localized “germ plasm” has been discovered. It contains RNA-rich granules and can be made visible with certain fluorescent dyes. The granules are tethered to the precursors of primordial germ cells. Fluorescent labels have made it possible to follow the entire tour of the wandering primordial germ cells. They are first seen in the ventral archenteron; from here they migrate in an amoeba-like fashion along the mesenteries (the epithelial ligaments by which the gut is suspended in the coelomic cavity). Eventually the primordial germ cells creep into the genital ridges, which will give rise to the gonads.
By no means is a granular “germ plasm” found in all animal organisms as an easily identifiable luggage. The RNA-containing granules that facilitate fluorescent tracking of germ cell precursors in Xenopus are missing from many animal groups, including newts, birds and mammals. To trace the path of germ cell precursors in these animals, other markers are used, such as monoclonal antibodies that attach to germ-line-specific surface molecules. Or the expression of germ line specific genes such as vasa (see Chap. 8) is visualized by in-situ hybridization and immunofluorescence (for techniques see in Box 12.2).
In the mouse embryo, primordial germ cells are first observed in the extraembryonic mesoderm; later they appear in the region of the allantois (Fig. 15.2). Subsequently, they creep along the allantois and gut toward the genital ridges.
In birds primordial germ cells are detected during neurulation along the anterior border of the blastodisc in a crescent-shaped endodermal zone (the germinal crescent). To reach the embryo, they use the blood stream for transportation over large distances. In this respect, they behave like lymphocytes and macrophages. But how do they know where to leave the blood vessels?
15.1.2 Blood Cells Emerge in the Vertebrate Embryo in Scattered Blood Islands
Blood cells originate from pluripotent stem cells, called hematopoietic stem cells (Chap. 18). In adult mammals, these are found in the bone marrow (in the mouse, in the spleen as well). However, the stem cells do not originate in these tissues; in the embryo, they arise anywhere in blood islands. In birds, blood islands are first observed outside the embryo proper in the area opaca where clusters of angiogenic cells form the first blood vessels. The peripheral cells of the clusters form the endothelial linings, whereas the central cells come to be blood cells. Many blood islands merge to form a capillary network.
In mammals, blood islands are detected anywhere in the extraembryonic mesoderm, especially in the mesodermal coatings of the yolk sac and in the region of the allantois. These locations coincide with places where associations of angiogenic cells form the first blood vessels (Figs. 17.2 and 17.3). Later, the stem cells of the blood cells colonize the liver, the spleen, the thymus and the bone marrow. In humans the bone marrow is the only place where new blood cells are generated lifelong. This will be considered in more detail in Chap. 18 (Figs. 18.4 and 18.6).
15.2 Neural Crest Descendants
15.2.1 Neural Crest Cells Migrate Into Many Target Areas and Are Endowed with Manifold Developmental Potentials
With the emergence of the vertebrates a new and astonishing rich and powerful source of founder cells came into play: the neural crest. As will be illustrated in Chap. 22 (Fig. 22.10) this source enabled the vertebrates to develop an elaborated head and efficient respiratory organs. The story of neural crest cells is among the most amazing tales in all of developmental biology. When the neural plate involutes to form the neural tube during neurulation, and the neural tube detaches from the ectoderm, a series of cells is left over on either side. The cells are neither integrated into the neural tube nor taken up into the ectoderm. Instead, they set off on travels, colonize various regions of the body and give rise to a bewildering variety of cell types, tissues and organs (Figs. 15.3, 15.4 and 15.5):
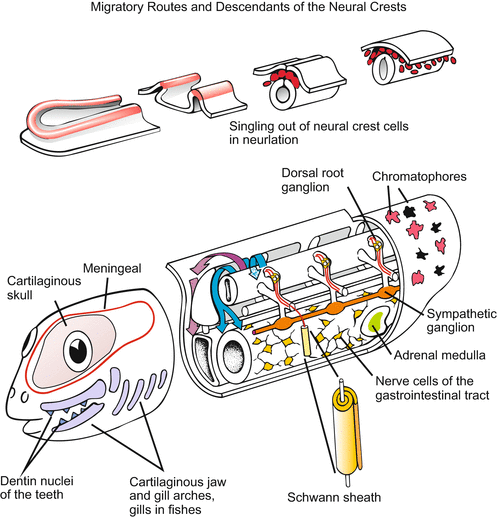
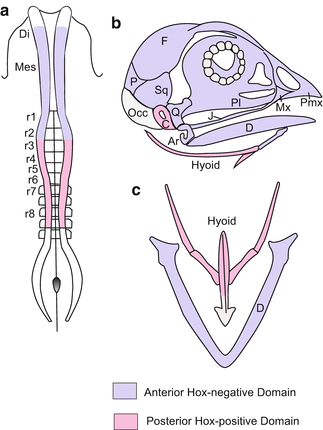
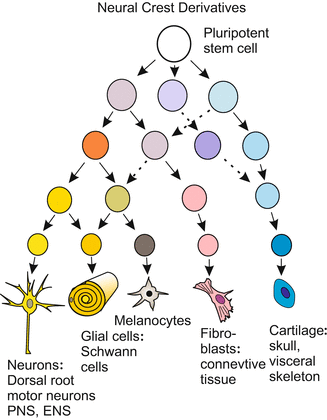

Fig. 15.3
Neural crest cells. Above: Singling out of neural crest cells during neurulation. Below: Posterior migratory route between dermamyotome and epidermis (red) and anterior migratory route between sclerotome and neural tube (blue), and cells/tissues derived from neural crest descendants

Fig. 15.4
Provenance of the cartilaginous elements of the skull and the visceral skeleton in birds, and relation to HOX-positive domains. (a) Neural crest viewed from above. Di Diencephalon, Mes Mesencephalon, r1 bis r8 Rhombomeres no.1 to no. 8. (b) Skull: Ar Articulare, C Columella, D Dentale, F Frontale, J Jugale, Mx Maxillare, Occ Occipitale, P Parietale, Pl Palatinum, Pmx Prämaxillare, Q Quadratum, Sq Squamosum. (c) Visceral skeleton: Hyoid = Zungenbein; D = Dentale = lower jaw. After Le Douarin et al. (2004)

Fig. 15.5




Pedigree (cell lineage) of the neural crest derivatives. Cephalic neural crest cells are endowed with the potency to give rise to fibroblasts and connective tissue of the head, among other cell types. After various sources
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


