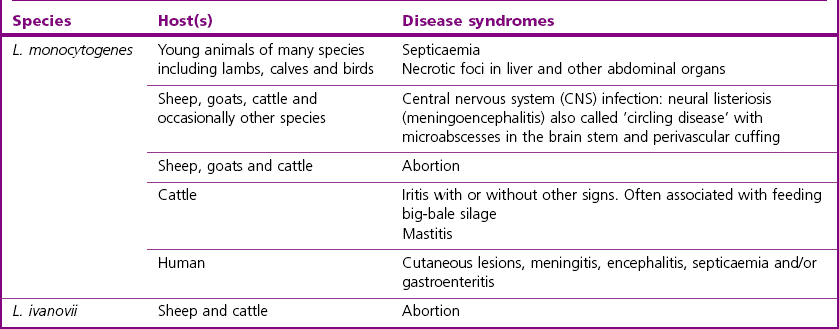
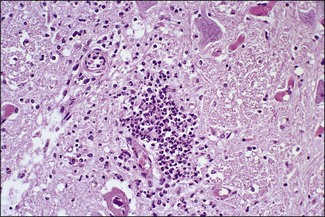
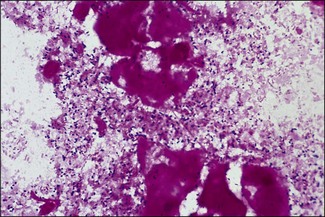
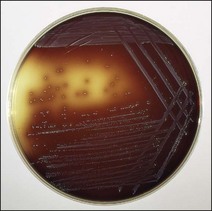
Chapter 12 The genus Listeria belongs to the Listeria-Brochothrix family which is a subline within the Clostridium subdivision. Six species form the genus Listeria: L. monocytogenes, L. ivanovii, L. innocua, L. seeligeri, L. welshimeri, and L. grayi; L. monocytogenes is the type species. Listeria ivanovii has two subspecies: L. ivanovii subsp. ivanovii and L. ivanovii subsp. londoniensis. Based on molecular phylogenetic studies, Listeria has also been divided into two distinct descent lines: (i) the closely related L. monocytogenes, L. ivanovii, L. seeligeri, L. innocua and L. welshimeri and (ii) L. grayi (Murray et al. 2007). Listeria species are medium-sized, non-branching, short Gram-positive rods, non-spore-forming and non-acid-fast. They usually occur singly or in a short chain. Listeria are facultative anaerobes but growth is enhanced by 10% CO2. They grow on nutrient agar and blood agar but not on MacConkey agar. Optimal growth temperature is between 30°C and 37°C but they can also grow at 4°C. They are usually catalase-positive, but exceptions are reported (Elsner et al. 1996), oxidase-negative, hydrolyse aesculin, tolerate 10% sodium chloride and are motile at 28°C due to a few (1–5) peritrichous flagella. Listeria monocytogenes and L. ivanovii are both pathogenic for animals. However, L. monocytogenes is by far the most significant pathogen causing septicaemia, abortion, mastitis, and central nervous system infection in various animals, mainly ruminants. L. monocytogenes also causes disease in man and is an important public health concern. Listeria grayi, L. seeligeri, L. innocua and L. welshimeri are considered to be non-pathogenic; however, there have been some reports of L. seeligeri causing illness in humans (Rocourt et al. 1986). Listeria species are widely distributed in the environment. Its primary habitat is considered to be soil and decaying vegetation where it survives and grows as a saprophyte. It can also be isolated from sewage, water, animal feed (silage), poultry, various meats, slaughterhouse waste, raw milk, and cheese (Fenlon 1999). Listeria species can grow in a temperature range of 3–45°C and within a pH range of 5.6–9.6. Silage is commonly implicated in outbreaks of listeriosis in cattle and sheep as the bacteria can multiply in silage with a pH over 5.5. Human foods frequently associated with listeriosis in man include coleslaw, soft cheeses, delicatessen items, milk, hot dogs, seafood and undercooked poultry meat. Listeria monocytogenes has been recovered from many species of mammals, fish, birds, crustaceans and insects. Asymptomatic faecal carriers of L. monocytogenes occur in man and animals (Fenlon 1999). It is likely that exposure of animals to L. monocytogenes is unavoidable because sources of the bacterium are numerous and the organism is hardy and persistent in the environment. Listeria monocytogenes can cause septicaemia, abortion, mastitis and central nervous system (CNS) infection (meningoencephalitis in adults and meningitis in young animals) in many animals but primarily in cattle and sheep. The septicaemic form of listeriosis is sometimes referred to as the ‘visceral form’ while central nervous system infection is known as the ‘neural form’. Table 12.1 indicates the hosts and disease syndromes caused by the pathogenic Listeria species, while major virulence factors of L. monocytogenes are presented in Table 12.2. The pathogenicity of L. monocytogenes is mainly determined by the virulence factors: listeriolysin O, protein ActA, phospholipases C, internalins (In1A and In1B) and a metalloprotease (Popowska & Markiewicz 2004). Listeria monocytogenesis is often employed as a model organism in studies on the pathogenesis of intracellular bacteria. It is able to penetrate, multiply and propagate in various eukaryotic cells and is capable of overcoming the three main barriers found in the animal host: the intestinal barrier, the blood–brain barrier and the placenta. Table 12.2 Main virulence factors of Listeria monocytogenes Listeria monocytogenes is a facultative intracellular bacterium. Factors that contribute to the virulence of L. monocytogenes can be divided into key events: attachment-invasion, intracytoplasmic growth and cell-to-cell propulsion (Gyles 2004). Listeria monocytogenes infections are initiated by its adherence, or attachment, to animal tissue cells. This is followed by invasion and internalization via the internalin A proteins (Cabanes et al. 2002). Both phagocytes and tissue cells can be infected. The bacteria escape from the phagosome via its haemolysin (listeriolysin O, LLO) and the phosphatidylinositol-dependent phospholipase C (Glomski et al. 2002). Once free in the cytoplasm, intracytoplasmic replication occurs leading to invasion of neighbouring cells. The method of cell-to-cell spread of L. monocytogenes is particular to this microorganism: deposition of host cell actin filaments on the end of the bacterial cells by the protein ActA promotes propulsion and invagination into neighbouring cells. The invagination process gives a double membrane around the bacterium. After the invasion of new cells, the process of phagosome escape starts again with lysis of the double membrane mediated by LLO and the phosphatidylinositol-dependent phospholipase C. Repetition of the entire process leads to a centripetal distribution of the microorganism. Listerial meningoencephalitis typically manifests first as depression and confusion followed by drooping of the ears and head tilt (Low & Linklater 1985, Low & Renton 1985) (Fig. 12.1). Salivation and tongue protusion are also usually seen while facial or throat paralysis and twitching may also be observed. The condition is referred to as ‘circling disease’ since the animal tends to move in one direction. The disease is associated with silage feeding and is seasonal, mostly occurring in winter and early spring when the animals are housed indoors on silage. Most pathogenic bacteria require the availability of iron in the host for their metabolic activities. It has been suggested that high iron levels in silage may predispose cattle and sheep to listeriosis. Entry of the organism is considered to occur via the dental pulp when sheep are cutting or losing teeth (Barlow & McGorum 1985) or, alternatively, through damaged mucosal surfaces and infection of the neural sheath of peripheral nerve endings of trigeminal nerves. The organism travels centripetally along the axons to the central nervous system. Histopathological lesions, usually unilateral, such as perivascular cuffing of mononuclear cells (Fig. 12.2) in the midbrain, pons or medulla oblongata and microabscesses (Fig. 12.3) are characteristic. Figure 12.1 Listeria monocytogenes: neural form of listeriosis in a silage-fed sheep showing unilateral facial paralysis. Figure 12.2 Perivascular cuffing in an ovine medulla indicative of the neural form of listeriosis. (H&E stain, ×400) Listeria monocytogenes also seems to have a tropism for invading the fetoplacental tissues of a variety of animals. Abortion usually occurs during the third semester of pregnancy. In sheep, abortion can be associated with septicaemia and encephalitis. In ruminants, the organism has been associated with generalized septicaemia and focal necrosis of the spleen and liver as well as mastitis. Cases of subclinical to severe suppurative mastitis have been described and treatment is reported to be difficult to achieve (Gitter et al. 1980). Because of its common recovery in bovine faecal material, Listeria is considered an environmental agent of mastitis (Weber et al. 1995). Clinical listeriosis is rarely seen in non-ruminant animal species such as pigs, chickens, dogs, cats and horses. It can cause septicaemia or meningitis (Cooper 1989, Schroeder & van Rensburg 1993). Keratitis has been reported in a horse (Sanchez et al. 2001). Listeria monocytogenes is considered as an opportunistic foodborne pathogen. Cases of human listeriosis may present as meningitis, encephalitis, septicaemia and/or gastroenteritis (Schuchat et al. 1991). Listeria monocytogenes infection in humans may also result in an influenza-like syndrome in pregnant women with infection of the foetus resulting in abortion or premature birth. The neural form of the disease can also occur in neonates. Listeriosis is also considered a zoonotic disease since veterinarians and abattoir workers can acquire a primary cutaneous listeriosis that infrequently leads to a generalized form of the disease. Stained smears are not as useful in cases of listeriosis as they are in some other diseases. Smears from lesions may reveal Gram-positive rods (often coccobacillary; Fig. 12.4) but isolation should always be attempted. Histopathological examination of fixed (10% formalin) brain tissue can often give a presumptive diagnosis of neural listeriosis due to the characteristic lesions. Traditional methods of identification involving culture methods based on selective enrichment followed by characterization of plated Listeria species on the basis of colony morphology, sugar fermentation and haemolytic activity are considered the gold standard. However, L. monocytogenes is widely tested for in food, environmental samples and clinical specimens. As a result, significant developments have occurred in selective enrichment procedures and many new and rapid detection methods using antibody and molecular-based technologies are now available (Gasanov et al. 2005). The use of a selective L. monocytogenes enrichment method is recommended for non-sterile specimens or foodstuffs to increase sensitivity and the recovery of injured Listeria cells. Selective enrichment methods involve the culture of specimens in a selective enrichment broth with inhibitors such as acriflavine and nalidixic acid designed to slow the growth of competitive organisms. A second round of enrichment is sometimes used and referred to as the two-stage enrichment procedure. Following incubation an aliquot of the broth is plated onto selective agar (Fig. 12.5) prior to biochemical identification of typical colonies. Reference methods are well described in the literature and have been the subject of review (Cassiday & Brackett 1989, Gasanov et al. 2005). A range of selective enrichment broths (Listeria Enrichment Broth (LEB), FDA Bacteriological and Analytical Method (BAM) formulation, full strength Fraser, half Fraser, polymyxin B-acriflavine-lithium chloride-ceftazidime-aesculin-mannitol (PALCAM)-egg yolk broth, University of Vermont Medium (UVM)) and selective agars (Oxford (OX), PALCAM, Modified Oxford (MOX), lithium chloride-phenylethanol-moxalactam (LPM)) are available. Figure 12.5 Listeria monocytogenes on ‘Listeria selective agar’. This medium is used for the detection of the bacterium in clinical specimens and foods. Hydrolysis of aesculin results in black zones around the colonies due to the formation of black iron phenolic compounds.
Listeria species
Genus Characteristics
Natural Habitat
Pathogenesis and Pathogenicity
Virulence determinants (gene)
Functions
Internalin A: 80-kDa surface protein (inlA)
Binds to E-cadherin on the basolateral surface of intestinal epithelial cells, a process involved in the invasion of intestinal epithelial cells
Internalin B: surface protein (inlB)
Cell invasion
Internalin C: surface protein (inlc)
Cell invasion
Internalin related protein (irpA)
Unknown role during disseminated infection
Listeriolysin O, LLO(hly): thiol-activated haemolysin
Lysis of phagosome membrane and escape into the cytoplasm
Phospholipase C, PC-PLC
Mediates bacterial cell-to-cell spread
Lysis of phagosome membrane and escape into the cytoplasm
Phosphatidylinositol-specific phospholipase C, PIPL-C: lecithinase
Mediates bacterial cell-to-cell spread
Lysis of phagosome membrane and escape into the cytoplasm
Actin-polymerizing protein ActA: 90-kDa surface protein (actA)
Directs the deposition of host-dependent actin filaments on the end of Listeria cells for propulsion into neighbouring cells
Positive regulatory factor A (prfA)
A temperature-, pH- and nutrient-regulated factor for Listeria virulence determinants
Invasion-associated protein (iap): major extracellular protein (p60)
Invasion
Metalloprotease (mpl)
Involved in the proteolytic activation of phospholipase C (PC-PLC)
Flagellin (fla)
Flagellar protein with murein-degrading activity
DegU regulator (DegU)
Pleiotropic regulator involved in expression of both motility at low temperature and in vivo virulence in mice

Laboratory Diagnosis
Direct microscopy
Isolation
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Listeria species
Only gold members can continue reading. Log In or Register to continue