Characteristics
Pediculus humanus capitis
Pediculus humanus humanus
Pthirus pubis
Lengtha of eggs
0.5–0.8m
0.6–0.8m
<1h
Durationb of embryogenesis
8g, 6–9m
7g, 5–6m
5–8g, 7–8i, 5–8m
Durationb of larval development
7–10f
7–10f, 8g, 13m
13–17i, 27m
Lengtha of males
2.4–2.6g, 2.4–2.9m, 2.0–3.0n
2–3k, 2.7–3.8m, 2.7–3.7n
1.3–1.6m
Lengtha of females
2.6–3.1g, 2.6–4.0m, 2.1–3.5n
3–4k, 3.0–4.8m, 2.7–4.4n
1.3–1.6m
Lifespanb of males
31e, 29m
Lifespanb of females
9f, 31.9l, 9–10 or 20–25m, 36n
34e, 9f, 30–40g, 30–60m
26g, 30m
Number of eggs/day
6f, 6.6l, 4–9m, 3–7n
9–10e, 12f, 5–14g, 5–7(10)m
3f, 1–3m
Number of eggs/lifespan
55f, 210l, 50–150m
270–300e, 110f, 300g, 200–300m
30g, 40–50m, 26–50n
Generation timeb (egg–egg)
21g, 17m, 17n
15g, 22–23m
25g
Maximal starvation capacityc (incubation temperatured)
55f(23), 30m(24)
85f(23)
24–48m, 26–42 (20)n
Temperatured preference
28–29n
31–33g, 31–33m
Lethal temperatured (incubation period, min)
50 (<30)g, 90–100 (1)g
The differences induced the search for morphological criteria. If glued to body hairs, head lice use more glue to connect the egg with the hair (Peters 1999; Mehlhorn and Mehlhorn 2009). P. humanus capitis is slightly smaller than P. humanus humanus (Table 11.1), but the lengths of the body overlap (Busvine 1978). In addition, in P. humanus capitis antennae, tibiae, and the second pairs of legs are shorter than in P. humanus humanus, and the abdominal segmental borders are laterally more distinctly separated (Busvine 1978; Habedank 2010).
Some molecular biological investigations supported a conspecific development, others, using sequencing of microsatellite DNA, the classification as separate species, and others indicated two genetically distinct lines developing to body and head lice or only head lice (Leo and Barker 2002, 2005; Leo et al. 2002, 2005; Reed et al. 2004; summarised by Li et al. 2010). A large-scale investigation of mitochondrial DNA markers shows more similarities between local populations of head and body lice than between populations of both subspecies originating from different countries, i.e., body lice arose several times from head lice (Leo et al. 2002; Li et al. 2010). Therefore, we follow the classification of body and head lice as subspecies.
11.3 Distribution and Morphology of Human Lice
Owing to the strong co-evolution, human lice are cosmopolitans. Since all species are wingless, the transmission requires an intimate contact or the common use of fomites such as combs and head gear (Habedank 2010). On the head and in the clothes of Eskimos and dark-skinned people, black head and body lice are present, not the normal pale beige or grayish head and body lice (Raoult and Roux 1999; Peters 1999) (Fig. 11.1), and in Brazil all intermediate colour forms of body lice can be found owing to reproduction of black and normal-coloured lice (Kollien unpublished).


Fig. 11.1
Light micrographs of a female of Pediculus humanus humanus about 3.5 mm in length during bloodsucking on human skin (a–d). The skin was black-inked to enhance the contrast of the micrograph. Light micrographs of typical beige (e) and black (f) morphs of P. humanus humanus (photographs by A.H. Kollien)
Head lice, P. humanus capitis, prefer the hairs on the neck and behind the ears, but in strong infestations they colonise all regions on the head and also eyelashes, eyebrows, and hairs in the armpit (Habedank 2010). They are mainly transmitted by head-to-head contact, and transmission is less likely by fomites such as combs and caps (Burgess 2004; Takano-Lee et al. 2005).
Body lice, P. humanus humanus, especially infest humans who rarely change and wash their clothes or possess only one set of clothes. Since lice react sensitively to high temperatures, body lice are more prevalent in regions with a temperate climate than in the tropics (Peters 1999). They prefer clothes, but in strong infestations they also colonise the hairs of the head (Habedank 2010). Body lice are adapted to the change to nighttime clothes and survive longer periods without a host than head lice. The morphology of both Pediculus ssp. is very similar (see Sect. 11.2.2), and the three pairs of legs are of similar lengths (Figs. 11.1 and 11.2a).
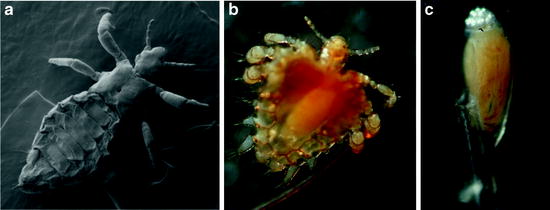

Fig. 11.2
Scanning electron micrograph of a male of P. humanus humanus (a). Light micrograph of an adult of Pthirus pubis gripping a human hair (b). Light micrograph of an egg of P. pubis glued to a human hair (c); the developing embryo is visible through the eggshell, and aeropyles for air and moisture supply of the embryo are located at the top of the egg (photographs by A.H. Kollien)
Crab lice, Pthirus pubis, are mainly transmitted during sexual contact; other modes of transmission seem to occur occasionally (Habedank 2010). The prevalence of crab lice might be reduced by the increasing shaving of hair in the genital region, but this species prefers the thickest hairs and thus also colonises the coarse hairs of the head and body, such as eyelashes, eyebrows, and hairs in the armpit (Peters 1999; Schaub 2008). The morphology of Pthirus differs strongly from that of Pediculus. In Pthirus, the thorax and abdomen are broader, and the legs stretch more sidewards, thus resembling the appearance of a crab (Habedank 2010). The first pair of legs are smaller than the second and third pairs (Fig. 11.2b).
Lice possess some morphological peculiarities: very short antennae (five segments), reduced eyes, no wings, and legs which have strong claws to cling onto the hairs of their hosts or fibers of the clothes (Schaub 2008). These curved claws at the end of the tarsus are opposite a thumb-like spine of the short tibia and thereby firmly grip hairs or fibers of clothes between them (Service 2004). In addition, the three segments of the thorax are fused, the end of a fusion tendency within the Phthiraptera (Dettner 1999). Also three segments of the abdomen are fused (Wenk and Renz 2003). The cuticle of lice is leathery and tough and not easy to crush. In both Pediculus ssp., females are slightly bigger than males. Females and males of P. pubis are of similar size and about 40% shorter than Pediculus ssp. (Table 11.1).
11.4 Biology of Human Lice
Lice spend their entire life cycle on the host and usually leave it only for transfer to a new host. However, if the host has a fever or dies, this induces a migration of the lice. All lice are highly host specific. Human lice will only infest some monkeys, and animal lice will not establish a population on humans (Habedank 2010). This has to be verified for the louse of gorillas, Pthirus gorillae, and the Pediculus sp. of monkeys, but the contact is too rare to be of importance. Transmission of head lice is supported by a behavioural peculiarity: After ingestion of blood, head lice go to the ends of the hairs, enabling an easier transfer. Some minutes later, they go back to the base of the hairs and remain hidden near the skin (Mehlhorn and Mehlhorn 2009). Of the three larval instars, females, and males of head lice, the first-instar larvae showed the lowest tendency to go to a new host, whereas adults were the most likely to disperse (Takano-Lee et al. 2005). Hungry body lice move towards the light (Hase 1915; Habedank 2010), and when engorged they hide close to the skin in the dark, especially between turnups at the seams of clothes, where they also prefer to lay eggs (Raoult and Roux 1999; Habedank 2010). Even exercise-induced increases of body temperature are sufficient to increase the activity of body lice (Habedank 2010).
Only once has a colony of body lice accepted a host other than humans (Culpepper 1944, 1946, 1948; Raoult and Roux 1999). These lice can be reared on rabbits or in vitro, and groups of both maintenance conditions show no strong differences in the development (Culpepper 1948; Habedank 2010). However, in attempts to maintain head lice in vivo and in vitro, development of membrane-fed older larvae was much slower and the fecundity of females and the hatching rate of their progeny were much lower (Takano-Lee et al. 2003a, b; summarised by Habedank 2010).
11.5 Development and Reproduction
The hemimetabolous development of lice includes the egg, three larval stages, and the adults. The eggs of the three human lice are similar in length, 0.5–0.8 mm (Table 11.1) (Burkhart et al. 2000; Habedank 2010). All are glued in a species- and subspecies-specific manner to the shafts of hairs, just above the skin, or fibers of clothes (Martini 1952). The glue is not dissolved by water or soap (Mehlhorn and Mehlhorn 2009). The cap-like operculum of the eggs points to the tip of the hair shafts or fibers of clothes and contains species-specifically arranged aeropyles for air and moisture supply of the embryo (Martini 1952; Burkhart et al. 2000). In P. humanus capitis and P. humanus humanus, the ventral side of the embryo is located at the hair, whereas the embryo of P. pubis is orientated laterally to the hair (Martini 1952). Eggs of the latter also possess a higher operculum and longer aeropyles than both Pediculus ssp. (Martini 1952; Peters 1999) (Fig. 11.2c). Like in all insects, the duration of embryonic development is temperature-dependent, lasting about 1 week under optimal conditions (Table 11.1) (Raoult and Roux 1999; Habedank 2010).
Like all terrestrial insects, the fully developed louse in the egg swallows air and forces the operculum off (Service 2004). After hatching, the cuticle of the first-instar larvae sclerotizes, and the larvae, which are 1-mm long, take the first blood meal (Raoult and Roux 1999; Habedank 2010). Usually they suck every 2–5 h, but body lice have to feed only at least once daily (Raoult and Roux 1999; Peters 1999; Habedank 2010). The duration of larval development is also temperature-dependent. Usually the molts to the second and third larval instars and to the adults occur 3, 5, and 10 days after egg hatching (Raoult and Roux 1999). Whereas head and crab lice live at relatively constant temperatures, the development of body lice is slowed if the daytime clothes are discarded at night.
Besides the smaller size, males of Pediculus can be distinguished from females by the rounded posterior end of the abdomen and the stylet-like penis in the sexual orifice (Fig. 11.2a), whereas at the end of the female abdomen a split appearance is caused by two large lobes that grip the hairs or fibers during egg laying (Ibarra 1993; Peters 1999; Service 2004). In addition, the dorsal side of the abdomen of males possesses dark transverse bands, and the forelegs are broader and bear a larger tibial thumb-like spine and tarsal claw than in females (Ibarra 1993). About 10 h after molting, adults copulate (Wenk and Renz 2003; Mehlhorn and Mehlhorn 2009). Whereas the female crab louse has a spermatheca to store the sperms of the male and presumably requires to be fertilised only once during its life, the two Pediculus ssp. lack this organ and have to be fertilised several times (Burgess et al. 1983; Raoult and Roux 1999). One day or 2 days after copulation, females start to lay eggs (Wenk and Renz 2003; Mehlhorn and Mehlhorn 2009; Haberkorn 2010). The number of eggs per day and the life spans of adults differ (Table 11.1), but body lice seem to live longer and produce more eggs than head lice, and both Pediculus ssp. lay more eggs than P. pubis. In body lice, the number of eggs per female is affected by the frequency of feeding (Gooding 1963). After membrane feeding using blood exclusively from women or men, there was no significant difference between the 24-h survivorships of the respective groups of head lice (Takano-Lee et al. 2005). The starvation capacity is species- and temperature-dependent. At 18 and 26°C, body lice survive on average for 35 and 24 days, respectively (Lang cited according to Burgess 2004). Near the optimum temperature, body lice survive longer than at lower and higher temperatures: at 6, 24, and 31°C all were dead after 6, 11, and 9 days, respectively (Mumcuoglu et al. 2006). However, even before movement ceases, they are unable to feed (Burgess 1995).
11.6 Haematophagy
The acquisition of blood as the sole food source causes many problems for ectoparasites. Temporary ectoparasites have to find a host, and all ectoparasites have to gain access to obtain the blood without being killed by defence reactions of the host. Finally, during and after blood ingestion, all are confronted with several defence systems of the host that have evolved against parasites, such as the complement system and antibodies, or the loss of blood via injuries, haemostasis (Andrade et al. 2005).
11.6.1 Biting and Blood Ingestion
Lice belong to the vessel-feeders, i.e., they ingest blood directly from a blood vessel (Dettner 1999). Their mouthparts consist of strongly modified labrum, labium, mandibles, and hypopharynx. A stylet is built from two half-pipes, the dorsal one originating from the hypopharynx and forming the blood channel and the ventral one originating from the labium. Between them or in the hypopharynx is the duct of the salivary glands. At the host, the labrum is pushed out of the head capsule in a hydraulic manner and contacts the skin (Peacock 1919). Then in alternating movements both parts of the stylet penetrate the skin. After a blood vessel has been pierced, blood is ingested. Pumping is performed by the cibarial pump and especially the pharyngeal pump. When blood ingestion has finished, muscles retract the mouthparts into the head capsule (Ramcke 1965; Dettner 1999; Wenk and Renz 2003).
11.6.2 Salivary Glands and Saliva
The two pairs of salivary glands, one pair reniform and the other bifurcated and tubular, lie in the anterior part of the thorax beside the pharynx (Peacock 1919). The saliva of lice is expected to store only limited amounts of protein (Mumcuoglu et al. 1996c). However, like in many vessel-feeders, the saliva of lice contains a vasodilator to increase the speed of blood ingestion (Jones 1998). Like all bloodsucking insects, lice have to overcome the normal haemostatic mechanisms of the host (Ribeiro 1987; Ribeiro and Francischetti 2003). The saliva of lice contains inhibitors of factor X and thrombin, thus preventing the synthesis of fibrin from fibrinogen. An inhibitor of thrombin also acts against one of the pathways of platelet aggregation; another one is inhibited by apyrases in the saliva (Mumcuoglu et al. 1996c). In contrast to triatomines (Meiser et al. 2010; Balczun et al. 2012), only a limited number of inhibitors have been identified, and in contrast to many dipteran bloodsuckers, lice possess no hyaluronidase, which is suggested to increase the permeability of tissues for other salivary components (Volfova et al. 2008).
11.7 Intestine, Blood Digestion, and Excretion
11.7.1 Intestinal Tract
The intestinal tract of lice is a relatively simple tube. The anterior midgut is a strongly distensible region and possesses two anterior caeca (Buxton 1947; Dettner 1999). It is followed by the narrow, less distensible posterior midgut. At the border of the hindgut, the four Malpighian tubules end. In the middle of the relatively long, narrow hindgut, six rectal papillae are located in a short, wide hindgut region (Buxton 1947; Dettner 1999).
11.7.2 Blood Digestion
Lice feed as permanent ectoparasites frequently, commonly every few hours, taking small blood meals. P. humanus capitis takes up only 0.19–0.3 times its unfed weight; females ingest on average 0.17 mg blood, males 0.07 mg, and nymphs 0.04 mg (Speare et al. 2006). Therefore, a heavy infection with head lice does not lead to clinically significant blood loss, even in iron-deficient people. If body lice are starved for 1 day, females and males ingest 0.9 and 0.3 mg blood, respectively (Martini 1952). In vitro fed females, males, and first-instar larvae suck 1.7, 0.7, and 0.08 mg blood, respectively (Habedank 2010). According to the frequent ingestion of blood, lice have the fastest digestion among haematophagous insects, about 4 h per blood meal, but the particular time for the digestion is always affected by several factors, such as ambient temperature, age of the insect, and mating status (Lehane 2005).
Like most haematophagous insects (Lehane 1991), lice use a range of alkaline digestive proteases. A leucine aminopeptidase of about 67–69 kDa has been partially characterised from the body louse (Ochanda et al. 2000). This enzyme has maximum activity at pH 8 and seems to be associated with the midgut epithelial cell membranes since homogenisation of midguts in the presence of detergent results in a tenfold higher aminopeptidase activity (Ochanda et al. 1998). If starved body lice are fed once in the laboratory, the leucine aminopeptidase is stimulated by a blood meal and reaches maximum activity 48 h after feeding. Under natural conditions, blood is ingested at short intervals and, therefore, the activity is supposed to be constantly stimulated and fairly constant (Ochanda et al. 1998). Partial purification of this enzyme indicates that only one aminopeptidase or at least a family of charged isomers of the same enzyme are present (Ochanda et al. 2000).
Trypsin-like enzyme activity appears in a laboratory experiment 24 h after feeding starved body lice once in the laboratory, i.e., much earlier than that of the aminopeptidases, and this activity disappears 48 h after feeding (Borovsky and Schlein 1988). Two trypsin-encoding genes (Try1, Tyr2) have been identified, and the Tyr1 gene seems to be expressed with identical levels in unfed first-instar larvae and fed adults. In addition, this gene is constitutively expressed in adults 2–24 h after a blood meal (Kollien et al. 2004). The expression of Tyr1, Tyr2, and an also characterized chymotrypsin-encoding gene (Chy1) has been localised in the distensible anterior region of the midgut (stomach) and—to a low extent—in the narrow posterior region of the midgut using whole-mount in situ hybridisation (Waniek et al. 2005). Tyr2 and Chy1 are also constitutively expressed for up to 24 h after blood feeding, the Tyr2 gene always being expressed at much lower levels that Tyr1 and Chy1.
The consecutive activities of aminopeptidase and trypsin after a blood meal indicate a network of different proteases is responsible for the digestive processes in the midgut of lice. A first hint at a regulatory network was given by the cleavage of an in vitro synthesised trypsinogen from P. humanus humanus by chymotrypsin leading to an activated trypsin (Kollien et al. 2004). Sequencing of the transcriptome and the genome of P. humanus humanus revealed the first insights into a putative network of digestive proteases (Pedra et al. 2003; Pittendrigh et al. 2006; Kirkness et al. 2010), and sequences for aminopeptidases, carboxypeptidases, trypsins, and chymotrypsins are now available in GenBank and VectorBase (http://www.vectorbase.org/). In future, the open access to such data will promote research on the digestion of lice since understanding of digestion is important for elucidating the basic biology of these insects, particularly with regard to their control as vectors of pathogens.
11.7.3 Excretion and Defecation
Like all bloodsucking insects, lice ingest a food in which water is the major constituent, about 80% (Lehane 2005); therefore, the Malpighian tubules secrete a proportion of this water. Body lice starved for about 1 day ingest so much blood that they defecate blood even during ingestion (Fig. 11.1d). In these populations, leakage of blood into the haemocoel also occurs, resulting in a transient red colouration (Raoult and Roux 1999; Kollien unpublished). This leakage seems to have no obvious effect on the survival (Kollien unpublished). During the normal period of digestion, lice defecate extremely dry and powdery faeces containing only 2% water (Burgess 1995). Similarly to the faeces of triatomines, the faeces contains a large amount of ammonium acting as an attractant for other lice (Raoult and Roux 1999; Schaub 2009).
11.8 Symbionts and Antibacterial Compounds of Lice
Like many bloodsucking insects, lice require symbionts for a normal development (Buxton 1947; Puchta 1955; Buchner 1965). Whereas in some of these insects the symbionts are located in the lumen of the intestine, tsetse flies and lice possess special organs, mycetomes, which are not connected to the intestine (Buchner 1965). Already in the first-instar larva, the mycetome is visible as a lenticular organ on the intestine in the middle of the abdomen. In males, this remains the only symbiont-bearing region. In third-instar larvae which will develop into females, all or nearly all symbionts migrate via the haemolymph to ovarial ampullae (Puchta 1955; Eberle and McLean 1982; Wenk and Renz 2003). From these proximal cells of the oviduct, the symbionts infect the egg via the micropyle and develop an infection mycetome in the egg yolk. In the developing embryo the midgut possesses a protrusion which is colonised by the symbionts and strangulated, thus losing the connection to the intestine (Puchta 1955; Wenk and Renz 2003). Immediately after hatching, the symbionts multiply in the mycetome. Aposymbiotic lice can be obtained by careful centrifugation of eggs. These larvae require supplementation of the blood with yeast or vitamins of the vitamin B complex (Puchta 1955).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


