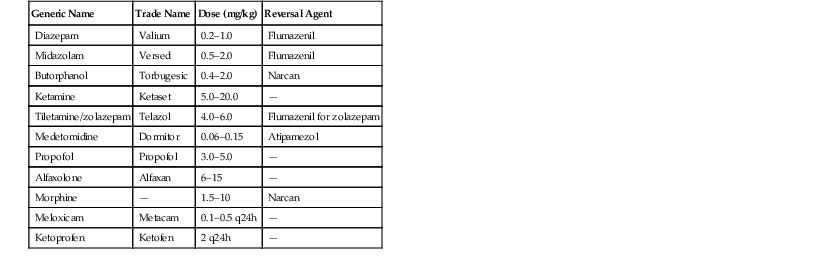
Ryan S. DeVoe The suborders Sauria/Lacertilia and Serpentes are found within the order Squamata, which contains between 6500 and 7000 species, depending on current taxonomic understanding. Approximately 5500 of the Squamates are assigned to the suborder Sauria/Lacertilia and are commonly referred to as lizards. A number of clades exist within the suborder Sauria/Lacertilia, including Iguania, Gekkota, Scincomorpha, Anguimorpha, and Amphisbaenia. These clades are further broken down into families, of which the Iguanidae, Agamidae, Varanidae, Scincidae, Chameleonidae, and Gekkonidae contain the vast number of species. The family Scincidae contains the largest number of species (approximately 20% of all lizard species), whereas other families such as the Helodermatidae contain only two species. Lizards are extremely successful reptiles and inhabit myriad habitats worldwide, ranging from desert to aquatic, temperate to tropical, fossorial to arboreal. Lizards have evolved to effectively take advantage of all these different habitats. The only continent on which lizards do not naturally occur is Antarctica. As would be expected with such a large and varied taxa, dramatic variations in anatomy, physiology, dietary strategy, and reproduction exist among species. Animals within the Sauria/Lacertilia range in size from the Komodo dragon (Varanus komodiensis), which may reach lengths over 3 meters (m) and weights exceeding 100 kilograms (kg) to some species such as the dwarf chameleons (Brookesia sp.) and geckos (Spaerodactylus sp.), which may not exceed 2 centimeters (cm) in length. Lizards are very popular as exhibit animals in zoos, aquaria, museums, and private collections. When properly managed, many lizard species are spectacular on display, hardy, and long-lived. Some species such as bearded dragons (Pogona vitticeps) and leopard geckos (Eublepharius macularis) have gained great popularity as pets and are propagated in large numbers to supply the pet trade. Relatively large amounts of information regarding the husbandry, reproduction, and medical care of these common species is available and may be judiciously extrapolated for use with similar species. Much variation exists in the anatomy and physiology in the Sauria/Lacertilia suborder; however, most lizards share a basic body form, and all others are variations on that theme. Most lizards have four well-developed legs and a tail. The dentition of most lizards is classified as either acrodont (agamids, chameleons) or pleurodont (iguanids). Acrodont teeth are fused to the biting edge of the mandibles and the maxillae. Pleurodont teeth are attached to the periosteum on the medial surface of the mandibles and maxillae. Clinically, this is significant, as pleurodont teeth will regenerate if lost or broken, whereas acrodont teeth will not.20 Some lizards such as monitors and tegus have long, forked tongues, which are very similar to those seen in snakes and used for tracking prey. True chameleons have very long tongues (over a full body length) with a sticky, fleshy tip that is used for capturing prey. The tongue is supported and propelled or retracted by specialized lingual muscles, the hyobranchial apparatus, and elastic collagen tissue. Most other lizards have fleshy, mobile tongues that are also used to prehend food and as chemosensory organs, but they are much less specialized than those in the taxa previously mentioned. In some species such as the common green iguana (Iguana iguana) the rostral portion of the tongue is dramatically different in color and appearance compared with the caudal portion, and the line of demarcation between the two regions is obvious, leading many clinicians to assume the presence of pathology, when this is, in fact, normal anatomy. Renal anatomy varies according to species, and exact location may be very significant to the clinician. In iguanids and some other species, the kidneys are found dorsally within the pelvic canal. In the normal animal, they are not palpable except via digital examination of the cloaca in a large enough specimen. In other species such as monitor lizards, the kidneys are found further cranial, well clear of the pelvic canal. Renal biopsies are relatively common procedures in lizards, so it is important to have a general idea of where the kidneys are normally located.16 Some lizard species have urinary bladders that communicate via a short, relatively wide urethra with the ventral aspect of the urodeum. The ureters deliver urine into the dorsolateral walls of the urodeum and do not connect directly to the urinary bladder when present. Many lizards (monitors, bearded dragons) do not possess urinary bladders and store urine in the colon, when necessary. Postrenal modification of urine in the bladder or the colon is possible, and the bladder serves as an important fluid storage organ in species from arid regions. All lizards have three-chambered hearts and are capable of shunting blood past the lungs to varying degrees. Much has been made of the clinical significance of the renal portal system, which allows for perfusion of the renal tubules when glomerular filtration is diminished, as might occur when the animal is conserving water. In the few studies that have been performed, administration of medications in the caudal portion of the body and passage through the renal portal system do not seem to affect drug pharmacokinetics in a clinically significant manner. Despite this fact, many clinicians still preferentially administer drugs, especially potentially nephrotoxic drugs, into the cranial portion of the body. 16 Some species (iguanas, geckos) are capable of tail autotomy, which is used as a predator avoidance strategy. Tails capable of autotomy reflexively fracture along specific planes and are cast with little to no hemorrhage. The tails will continue to wiggle vigorously after being cast, and ideally distract the predator, allowing the lizard to escape. The veterinary clinician should recognize which species are capable of casting their tails, as some will do so in response to even careful handling or simply to severe stress. Other lizard species have prehensile tails (chameleons, certain skinks), which aid in living an arboreal lifestyle.20 For many years, the members of the family Helodermatidae were the only lizards identified as venomous. In recent years, venom and associated glands or delivery systems have been identified in a number of lizard species, including monitors and agamas. Most notable is the Komodo dragon, which historically was thought to incapacitate prey by inducing septic shock via transfer of oral bacteria to bite wounds. It has been shown that it is a potent venom produced by the lizard, not bacterial toxins, that can dramatically impact blood pressure and hemostasis, incapacitating the prey following a bite. The venom delivery system in lizards differs from those in snakes in the location of the venom glands (along the mandible in heloderms) and the presence of many ducts that release venom at the base of teeth versus the single ducts and hypodermic-like fangs of snakes.11 The gastrointestinal (GI) tract is fairly simple in most species of lizard. A simple stomach and relatively short, unconvoluted intestinal tract are commonly found. Some herbivorous species such as green iguanas are hind-gut fermenters and have well-developed cecae and large sacculated colons.6 Much of a captive lizard’s health depends on provision of a proper environment. It is critical that configuration of the captive environment reflect the natural history of the animal. Temperature, light, humidity, and ventilation, as well as the size, spacial orientation, substrate, and cage furniture, are all extremely important parameters that need to be considered when creating the optimal lizard habitat. Many species will survive and even reproduce in spartan accommodations consisting of little more than a plastic box with newspaper substrate, a water bowl, and a hide box. However, a more recent trend is toward the provision of more naturalistic accommodations for all captive reptiles, including lizards. The animals are more visually pleasing when accommodated in naturalistic environments, and they also are more likely to exhibit a wider variety of behaviors that one might not see if the animals were provided with a less stimulating environment. Lizards, like most nonavian reptiles, are ectothermic and behaviorally thermoregulate by movements within their environment. Ectothermic animals vary their body temperatures according to the prevailing metabolic need, so to function properly, they require access to a thermal gradient within their preferred temperature zone. Gradients are also important with other parameters such as light and humidity. Minimum acceptable size for a lizard enclosure varies according to species with regard to not only the size of the lizard but also the activity level and flight distance. Bigger is typically better in most cases. The size of the enclosure should allow the animal to move about freely and accommodate creation of multiple comfortable resting spots for the animal. Obviously, the orientation of the cage should reflect the habits of the lizard. Arboreal species should be provided with a vertically oriented enclosure with appropriate climbing structures. Terrestrial and burrowing species should be given a horizontally oriented habitat with plenty of appropriate substrate. It is important that the lizard be able to choose its own appropriate environmental temperatures, humidity, and light exposure while feeling safe and secure. Many lizards will choose security over proper temperature, so basking spots need to be secure enough for the animals to use them properly. In a similar fashion, some lizards will choose thermal needs over the need for exposure to ultraviolet B (UVB) rays. Therefore, if possible, the artificial light and heat source should be combined. Substrate may vary, including artificial materials such as newspaper and indoor–outdoor carpeting. An overriding concern with any captive lizard enclosure is hygiene. Regardless of how beautiful and functional the habitat is otherwise, it if it cannot be properly serviced, it is useless and potentially harmful to its inhabitants. Prior to the capture and physical restraint of a lizard, a basic plan should be in place to minimize handling time, and all necessary equipment should be assembled prior to getting the animal in hand, if possible. Also, care should be taken to provide secondary containment, if possible, in case the animal evades capture and escapes the primary enclosure. Lizards are capable of inflicting damage on a handler through bites, scratches, and tail lashes. The size and conformation of the species determines the risk involved. A house gecko (Hemidactylus sp.) bite may be essentially ignored, but a bite from a species such as a crocodile monitor (Varanus salvadorii) may warrant a trip to the emergency room and may even result in permanent disability. It is important to know the species’ behavioral profile when planning a capture-and-restraint episode. For instance, it is helpful to know that many monitor species will initially defend themselves by delivering surprisingly accurate tail lashes. If that does not deter the person attempting the capture, they will quickly resort to biting and scratching. Most lizards may be safely restrained by gaining control of the head and neck with one hand and the pelvic area with the other. With large and aggressive animals, the judicious use of towels and leather gloves may help facilitate capture and restraint and provide an extra measure of safety. Very large lizards such as Komodo dragons, water monitors, and crocodile monitors may require multiple personnel or chemical restraint to work with safely. On the other end of the spectrum are very small or fragile species that pose no threat to the handler but are easily injured if extreme care is not employed. Certain gecko species have extremely thin and delicate skin, which may be torn as they struggle to escape restraint. Some species with tail autotomy will drop their tails, even if the handler is not actually touching it. There is also the possibility of other injuries associated with attempted or successful escapes from handlers, including fractures, crushing wounds, and so on. When working with these very small, delicate species, it is often easiest to perform most of the examination with the animal inside a clear container or anesthetized. A strategy that may be used with small geckos or other lizards is to induce anesthesia with an isoflurane or sevoflurane-soaked cotton ball in a sealable plastic bag. Most of a physical examination may be performed with the animal in the bag, and if venipuncture is required, the tail may be accessed by cutting the corner off the bag to exteriorize the tail. Care should be taken to work quickly with this method, as gas anesthetic levels created are quite high and could result in an overdose. Frequent handling of some species of lizard (and many other taxa for that matter) may result in significant stress, which may affect the general health of the animal. True chameleons are notorious for this, so in most situations, handling should be kept to a bare minimum. Some captive animals may seem very docile, bonded to their keepers and not at all perturbed by handling; however, these individuals are the exception to the rule. The nutritional requirements of the animals with the sub-order Lacertilia are incredibly varied. Lizards may be carnivores, insectivores, herbivores, or omnivores. Additionally, many species have very specific nutritional needs, being adapted to exploit particular resources in the wild. For instance, the caimen lizard (Draecena guianensis), a large and attractive species from South America, feeds exclusively on snails in the wild. Other specialized feeders include the horned lizards, which prey exclusively on ants and termites, and marine iguanas, which feed on marine vegetation. These specialized feeders create challenges in situations of captivity. Sometimes the preferred food item may be replaced with items more readily available with good results. However, in most situations, the animals will do poorly and refuse to accept anything but the preferred foodstuff. A difficult issue to address in captivity is that with the exception of the specialized feeders, most wild animals are probably consuming a large variety of food items that are not readily available in captivity. Most captive animals receive very little variation in their diets, which may lead to unrecognized deficiencies. Obesity is a common nutritional disorder in captive lizards. Captive animals expend much less energy than their wild counterparts, and well-meaning lizard keepers tend to enjoy interacting with their animals by feeding them. The consequences of obesity in lizards and reptiles in general are thought to mirror those seen in mammals, and clinical experience would suggest that this is true. Orthopedic disease, cardiovascular disease, and GI and reproductive dysfunctions all seem related to obesity in captive lizards. Care should be taken to limit caloric intake and maximize physical activity in captive animals to avoid the development of obesity. Data from wild animals may be used to guide weight management in captivity.9 It is important to remember that most wild animals appear too thin to the average keeper! Chemical immobilization may be required when an animal is potentially dangerous to the handlers or the procedure requires an immobile patient or is likely to induce pain. Many options exist for anesthesia in lizards (Table 7-1), and the choice of a plan for anesthesia should be based on the species, the animal’s condition, and the reason for the procedure rather than adopting a “one size fits all” approach. Preanesthesia evaluation and planning are critical pieces of the puzzle, as it is unwise to attempt to anesthetize an animal with severe physiologic derangement unless absolutely necessary. Lizards, like most other reptiles, are sometimes capable of withstanding changes in fluid and electrolyte balance, circulation, and so on that would quickly result in the death of most mammals. Even though these animals may withstand these insults to a point, anesthesia may be the proverbial “straw that broke the camel’s back” and result in the death of an animal that may have been able to recover if given proper support. Propofol is a common choice for induction of anesthesia in lizard species as long as intravenous or intraosseous access is possible. Use of propofol in lizard species is typically associated with very rapid onset of effect and recovery. Propofol may be used as a sole anesthetic agent through continuous rate infusion or delivery of repeated boluses.1 In recent years, alfaxolone has become available in some countries and is in the process of evaluation for distribution in the United States. Alfaxolone has properties very similar to propofol with regard to induction, recovery, and minimal effect on cardiopulmonary status in lizards but has the very important added benefit of being effective when administered intramuscularly. When more widely available, alfaxalone will likely become the preferred anesthesia induction agent in most reptiles, including lizards.2,31 Ketamine is still a viable choice of injectable anesthetic for lizards, especially large and dangerous animals that may require remote drug delivery. However, induction and recovery times with ketamine may be quite unpredictable and prolonged. The effect of ketamine may be enhanced by addition of an α-2 agonist or benzodiazepine (diazepam or midazolam). Tiletamine-zolazepam may also be used effectively and usually results in rapid inductions, but recovery time may be extended. Isoflurane and sevoflurane are frequently used for induction and maintenance of anesthesia in lizard species. Induction using inhalants may be difficult and prolonged, as lizards as well as other reptiles are capable of shunting blood away from their lungs and hold their breath for extremely long periods without any adverse effect. Rapid anesthesia induction for very short procedures such as venipuncture in diminutive species may be accomplished by placing the animal in an airtight container with a cotton ball soaked in isoflurane or sevoflurane. The resultant percentage of agent to which the animal is exposed is extremely high, so the animal should be monitored very closely and removed from the container as soon as adequate anesthesia is achieved. Small lizards may easily be euthanized via this method simply by leaving them in the container for an extended period. Monitoring the physiologic parameters of lizard patients during anesthesia is important; however, critical limits to measured parameters are unknown in most cases. Nonetheless, it is probably best to try to maintain parameters such as heart rate, respiratory rate, and temperature as stable and close to what would be seen in a healthy, awake animal as possible. Much of the currently available monitoring equipment may be used with the larger lizard species. However, some lizards are so small that even a fingertip Doppler probe is too big for them. Caution should be used to not overinterpret the measurements delivered by equipment that has not been validated for use with the species in question. For instance, oscillometric blood pressure measurement via cuff on a hindlimb does not work well or correlate with direct arterial pressure measurements in the green iguana.3 In recent years, a number of studies have investigated the effectiveness of certain analgesics in lizard species, although most of the information available regarding the use and dosage of the majority of drugs is anecdotal. Morphine has proven to provide analgesia in bearded dragons,33 and clinical impressions suggest that it is effective in other species at similar doses. Butorphanol has been evaluated by different researchers in the green iguana, with different conclusions regarding efficacy. However, in these studies, the observed result could be explained by differences in methodology. Other opioid medications that target the same receptors as morphine should theoretically be efficacious; however, they have not been evaluated, and dosing would be empirical at best. Nonsteroidal anti-inflammatory medications such as meloxicam and ketoprofen are frequently used in lizard species, and some pharmacokinetic data exist to guide usage.7,35 It is critical to understand that the most useful diagnostic in any case is a thorough history and physical examination. A careful, systematic physical examination with an understanding of normal anatomy, conformation, and behavior is one of the first steps in proper case management. Appropriate use of light and magnification may aid an examination infinitely, especially in small specimens. In small species with thin skin, transillumination with a cool light may yield tremendously useful information. Imaging using the various available modalities may be useful in the medical management of lizards.32 Radiography is very commonly used, as it is available to most practitioners. The imaging of skeletal structures is fairly straightforward but may be difficult in very small specimens unless high detail systems are available. Lizard species with osteoderms or heavy scales may be difficult to radiograph, as their dermal structures do not allow for proper imaging of internal structures. Identification of soft tissue structures is possible with proper knowledge of normal anatomy. Techniques employing positive and negative contrast media may be useful in certain instances. The application of ultrasonography in the evaluation of lizard cases is becoming increasingly common as appropriate equipment becomes more readily available and clinicians gain confidence with the modality.34 Since lizards may vary so dramatically in size, probes with various footprint sizes and shapes are necessary to accommodate the possible range of species. Small transducer probes capable of emitting frequencies in the 10- to 12-megahertz (MHz) range are extremely useful in small species. With very small specimens, a standoff, which may be purchased or simply manufactured by filling a latex glove with water, is extremely useful and allows for more complete imaging of the patient’s anatomy. Coupling of the transducer probe with the sometimes heavily scaled skin of a lizard patient may create challenges. Gel may be effectively employed as a coupling agent, but often this leaves air bubbles under and around the scales creating artifacts that make evaluation difficult. Performing the examination with the lizard’s body submerged in a tub of water may be an effective method of creating adequate transducer coupling. Combined with knowledge of the normal anatomy of the species, ultrasonography may be a very effective and useful tool for evaluation of soft tissue structures and facilitate collection of diagnostic samples via aspiration. Computed tomography (CT) and magnetic resonance imaging (MRI) are also used occasionally with lizards and may dramatically aid a diagnostic investigation if applied correctly. Many veterinary facilities house CT scanners that are capable to creating useful images, even in very small patients. Software that creates three-dimensional reconstructions of CT images is available. These reconstructions are especially useful, as they allow the clinician to visualize the anatomy of the region or structure in question thoroughly from all angles. When evaluating soft tissue change, MRI is superior to CT, so in cases specifically evaluating the central nervous system or coelomic organs for mass lesions, MRI is the preferable modality. 32 Because of lizards’ stoic nature and ability to withstand significant insult without resultant changes in hematology or plasma biochemistry parameters, it is often necessary to directly visualize and collect biopsies of diseased tissue to make a diagnosis. Minimally invasive surgical techniques with endoscopy are frequently employed to assess lizards and collect samples.8 These techniques are useful even in extremely small patients, as telescopes in the 2- to 3-millimeter (mm) range are available. Coelomic structures, as well as those in the GI, respiratory, and reproductive tracts, are all accessible via endoscopy. In addition to collection of diagnostic samples, endoscopy is also frequently used to determine sex in monomorphic species and to perform certain surgical procedures. Collection of blood for hematology and plasma biochemistry analysis is a routine procedure in the evaluation of lizard patients.29 It is generally accepted that an amount of blood of between 0.5% and 0.8% of body weight in grams may be collected safely in most lizard patients. Proper handling of the sample is important to obtain the most accurate information possible. Lithium heparin is the most commonly used anticoagulant in reptile species; however, ethylenediaminetetraacetic acid (EDTA) is considered the anticoagulant of choice in some species such as the green iguana and Chinese water dragon. Heparin may create clumping of leukocytes and thrombocytes and cause a blue cast to blood films, which makes evaluation difficult. Therefore, it is recommended that blood films be made prior to placement of the sample in the anticoagulant or very soon thereafter. A number of reference ranges have been published for various species (Tables 7-2 and 7-3), but it is important to understand that these values rarely represent true “normal reference ranges.” Nonetheless, these reports may serve as a starting point for evaluation of clinical cases. Diagnostic blood samples from lizards are usually collected from the ventral coccygeal vein, but other options include the ventral abdominal vein, the jugular vein, and the cranial vena cava.28 Samples for blood gas analysis may be collected from the lingual veins, as the blood in these vessels approximates arterial samples. TABLE 7-2 Reference Ranges for Hematologic Parameters of Selected Lizard Species
Lacertilia (Lizards, Skinks, Geckos) and Amphisbaenids (Worm Lizards)
Biology and Taxonomy
Anatomy and Physiology
Special Housing Requirements
Physical Restraint and Handling
Feeding
Anesthesia and Analgesia
Diagnostics and Laboratory Sample Collection
Parameters
Tegu Lizard
Caimen Lizard
Egyptian Spiny-Tailed Lizard
Frilled Lizard
Mexican Beaded Lizard
Green Iguana
Prehensile-Tailed Skink
Crested Gecko
Gila Monster
Erythrocytes (×106/µL)
0.8 ± 0.2
0.7 ± 0.2
0.9 ± 0.4
1.85 ± 2.62
1.4 ± 5.8
1.45
0.50 ± 0.13
PCV (%)
35 ± 7.5
32 ± 5.8
26 ± 6.3
40 ± 8.9
32 ± 5.9
38 ± 52
35
Male 36.2 ± 4.8
Female 30.6 ± 4.6
37 ± 8
Hemoglobin (g/dL)
9.1 ± 0.3
4.6 ± 1.4
9.5 ± 3.7
9.7 ± 1.4
11.7 ± 18.6
9.6
7.4 ± 0.9
MCV (fL)
424.7 ± 156.4
324.2 ± 61.6
519.2 ± 213.6
326.7 ± 182.3
263
812 ± 370
MCH (pg)
184.7 ± 76.7
86.6 ± 11.7
±
144.1 ± 56.0
69
±
MCHC (g/dL)
38.6 ± 0
23.4 ± 4.7
34.8 ± 20.0
31.7 ± 4.8
28
20.9 ± 5.3
Leukocytes (×103/µL)
17.7 ± 13.2
10.6 ± 7.2
12.6 ± 7.9
17.9 ± 14.2
5.5 ± 4.5
1.7 ± 15
12.4
15.4 ± 7.1
4.72 ± 0.84
Heterophils (×103/µL)
6.4 ± 3.8
3.6 ± 3.4
9.1 ± 6.8
9.0 ± 7.2
2.0 ± 1.6
5%–55%
4.4
3%–39%
2.17 ± 0.61
Lymphocytes (×103/µL)
9.1 ± 8.0
1.9 ± 1.0
1.9 ± 1.3
6.5 ± 6.4
2.2 ± 2.4
33%–61%
2.7
10.7 ± 5.1
1.54 ± 0.8
Eosinophils (×103/µL)
0.3 ± 0.2
0.8 ± 0.5
0.1 ± 0.0
0.3 ± 0.2
0.3 ± 0.4
0%–1%
0.6
0%–2%
0 ± 0
Monocytes (×103/µL)
1.4 ± 1.1
1.9 ± 2.5
1.8 ± 1.7
0.9 ± 1.0
0.3 ± 0.4
12%–35%
0.1
6%–33%
0.07 ± 0.08
Basophils (×103/µL)
0.6 ± 0.6
3.0 ± 2.1
0.7 ± 1.1
0.4 ± 0.4
0.8 ± 1.1
5%–11%
1.0
0%–12%
0.57 ± 0.23 ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Lacertilia (Lizards, Skinks, Geckos) and Amphisbaenids (Worm Lizards)
Chapter 7