Chapter 81 Johne’s Disease and Free-Ranging Wildlife
Cause
The mycobacterial organism causing Johne’s disease in ruminants belongs to the Mycobacterium avium complex (MAC), and is now broadly referred to as a subspecies of M. avium, itself a ubiquitous opportunistic pathogen for humans and animals alike. The members of this mycobacterial complex, previously classified phenotypically but now under reclassification via molecular typing methods, are acid-fast, slow- to very slow-growing, nonpigmented, rod-shaped bacteria with complex, lipid-rich cell walls.27 The elaboration of MAP strain K-10’s genome in 2005 sparked a proliferation of molecular typing analyses in concert with the elucidation of the complementary genome of its cousin, M. avium subsp. hominissuis.13,19 These studies have parsed MAP isolates into numerous strain groups beyond the S (sheep) and C (cattle) categories previously used. These strain discriminations are not diagnostically critical for wildlife managers, although analyses of the genetic polymorphisms and potential virulence variations across strains that have been revealed are valuable from a research standpoint, because MAP strain types are not restricted to host species—for example, so-called sheep strains have been isolated from cattle, and cattle strains from bison, sheep, and deer. Each strain is capable of infecting and eventually causing disease in ruminants and each strain may be isolated through culture by an experienced laboratory. In the United States, the organism’s genome is highly conserved, with most (78%) isolates from cattle belonging to the same genetic node (e.g., the cattle strain now referred to as type I).17 For wildlife managers, a reasonable working hypotheses is that any strain of MAP may infect any ruminant host, the organism may be subsequently shed by an infected animal in milk and feces, and any such infection will eventually kill the ruminant host.
MAP is resistant to heat, cold, drying, and acidic conditions, but does not replicate in the environment. An obligate pathogen, MAP in the environment does eventually die off completely, but not quickly. When a premise is contaminated by MAP-containing manure, approximately 90% of the organisms are believed to die off within a few months, but MAP in low numbers may be recovered for more than 1 year. The relevance of this persistence at low levels to new cases of infection is unknown. In Australia, MAP could be isolated (albeit from fewer and fewer samples over time) from shaded soil (including soil shaded by crops) for up to 55 weeks, and in shaded trough water at 48 weeks. Even greater longevity was noted in trough sediment. MAP was isolated from grasses germinating through manure-laden soil, again in the pattern observed for MAP isolation from soil; greater MAP longevity was seen in grasses grown from completely shaded versus 70% shaded soil boxes (24 versus 9 weeks).28 This study’s comparison of shaded versus partially shaded sites led the authors to conclude that diurnal temperature fluctuation was more relevant in hastening complete MAP die-off than was ultraviolet (UV) radiation, and removal of vegetation to maximize temperature changes at soil level may be beneficial. Based on these data, contaminated drinking water may remain a reservoir for new infections longer than contaminated ground.
Epidemiology
The primary route whereby wildlife may encounter MAP is by sharing range with infected domestic agricultural ruminant species. Because of long-standing patterns of husbandry (e.g., high animal density, bottle-feeding pooled milk and colostrum, fecal contamination of calf water, premises, and feed), in addition to a history of low biosecurity trade across states and countries, the global dairy industry reports the highest prevalence of Johne’s disease. The U.S. Department of Agriculture recently stated that the organism was detected on 68% of U.S. dairy premises (based on pooled environmental sample culture data; http://www.aphis.usda.gov/vs/ceah/ncahs/nahms/dairy/dairy07/Dairy07_is_Johnes.pdf). Given that a cow produces 100 pounds of manure daily and that manure spreading on fields is a common agricultural practice, the environmental (soil and water) burden of MAP is highest in intensively farmed areas. Browsing ruminants are likely primarily at risk through shallow sources of contaminated water and grazing ruminants through both contaminated water and grasses in these areas.
Ruminant Infections
Clinical Signs
After infection, the clinical disease known as paratuberculosis is slow to develop; affected wild ruminants are generally 1 year of age and usually much older. The vague and nonspecific primary clinical signs are loss of body condition, poor hair coat, and diarrhea in the later stages of the disease, which may be intermittent and may not appear at all in some species (Fig. 81-1).
Pathology
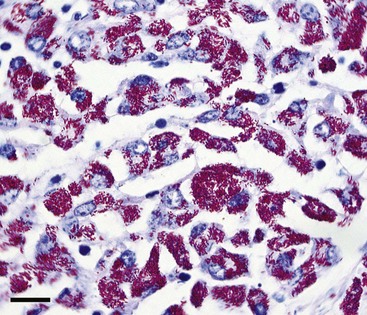
Lesions may range from inapparent to florid, depending on the stage of infection at the time of necropsy and the species being examined. For example, in some cases of infection in sheep, bison, and perhaps other nondomestic species, the gastrointestinal tract may appear completely normal, even when the animal is clinically affected. At the other extreme, the ileum may be thickened, corrugated, and reddened, with dilated lymphatic vessels coursing over its serosal surface as well as the serosa of enlarged mesenteric lymph nodes. Clinically ill animals are often emaciated, with a total absence or necrosis of abdominal fat stores. Microscopically, acid-fast rod-shaped organisms (scarce to numerous) may be noted within macrophages as part of a granulomatous infiltrate (Fig. 81-2). This inflammation may be slight or may completely efface ileal villi.
Bison
Several articles in the literature have addressed Johne’s disease in bison in the United States.2 Each article relied on the same samples, herd history, and isolates obtained from a single population in Montana. Unfortunately, extrapolations from this population to general conclusions about MAP in bison (e.g., that MAP in bison is unusually difficult to detect via culture or that serology is ineffective) have turned out to be inaccurate, at least for bison in other parts of the country. Several but not all the commercial enzyme-linked immunosorbent assays (ELISA) detect antibody in bison and, in numerous cases, the MAP strain has been easily isolated and characterized as the same that is detected in other U.S. species.17 Clinical signs and pathologic lesions mirror those seen in cattle and transmission of the organism is the same. In Canada, surveillance of bison with the polymerase chain reaction (PCR) assay produced positive results in several herds, but the organism was not recovered. A serologic survey of banked sera collected over multiple years from free-ranging bison managed in four western national parks was completed under the auspices of the National Park Service. ELISA results for more than 1200 serum samples did not indicate the presence of MAP infection in these populations.15
Deer and Elk
Infected tule elk (Cervus elaphus nannodes) were first detected in 1979 at the Point Reyes National Seashore (PRNS; Marin County, Calif). The infection has been confirmed in adult animals since that time.16 Reserve managers have reported no obvious effect on herd health or reproduction in the approximately 450-member herd because of Johne’s disease; diagnostic testing is no longer part of the management plan.8 No evidence of MAP infection has been found in native black-tailed deer (Odocoileus hemionus columbianus) also located at PRNS. Although an infection prevalence in two non-native cervid species—axis deer (Axis axis) and fallow deer (Dama dama)—sharing the range with tule elk was estimated at 8% to 9% 20 years ago, the infection appears also to have had minimal impact on population-based health parameters. Surveys in Arkansas4 and Montana, and in Wyoming elk,22 did not reveal indicators of MAP infection in these free-ranging populations.
The causative organism has also been isolated from tissues of clinically normal free-ranging white-tailed deer (Odocoileus virginianus).5 However, clinical disease as a result of infection with MAP in free-ranging white-tailed deer is rarely reported as opposed to several reports of clinical cases in captive white-tailed deer.10,14 Paratuberculosis was first diagnosed in an endangered Florida Key deer (a subspecies of white-tailed deer; O. virginianus clavium) in 1996 and later in six additional Florida Key deer from 1998 to 2004. Subsequent surveys have indicated that the organism persists in the Florida Key deer population and environment at a low prevalence, but its distribution is limited to a relatively small geographic area within their range.20 A single clinical case in a 2-year-old male white-tailed deer from Virginia has also been reported.26 Subsequent surveillance failed to reveal additional cases or infected animals. The authors concluded that infection with MAP is an infrequent occurrence in these deer, and speculated that a local cattle farm was the likely source of infection. A study that performed multistate surveys of wild white-tailed deer in the southeastern United States also revealed a very low prevalence of infection (0.3%).5 It thus appears that white-tailed deer do not currently constitute a broad regional reservoir for this organism.
Clinical Johne’s disease has also been reported on occasion in other free-ranging ungulate species, including Rocky Mountain bighorn sheep (Ovis canadensis canadensis), Rocky Mountain goats (Oreamnos americanus), and free-ranging red (Cervus elaphus hippelaphus) and fallow deer in Europe.9
A quicker progression from infection to clinical signs has been reported in farmed cervids (red deer or elk) than bovids, with animals younger than 2 years of age rapidly losing weight, developing severe pathology, and dying of the infection.6,14 Whether this is a function of stress, dose, or other factors caused by high animal density under intensive husbandry as opposed to a factor innate to cervids’ response to MAP is not known.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree