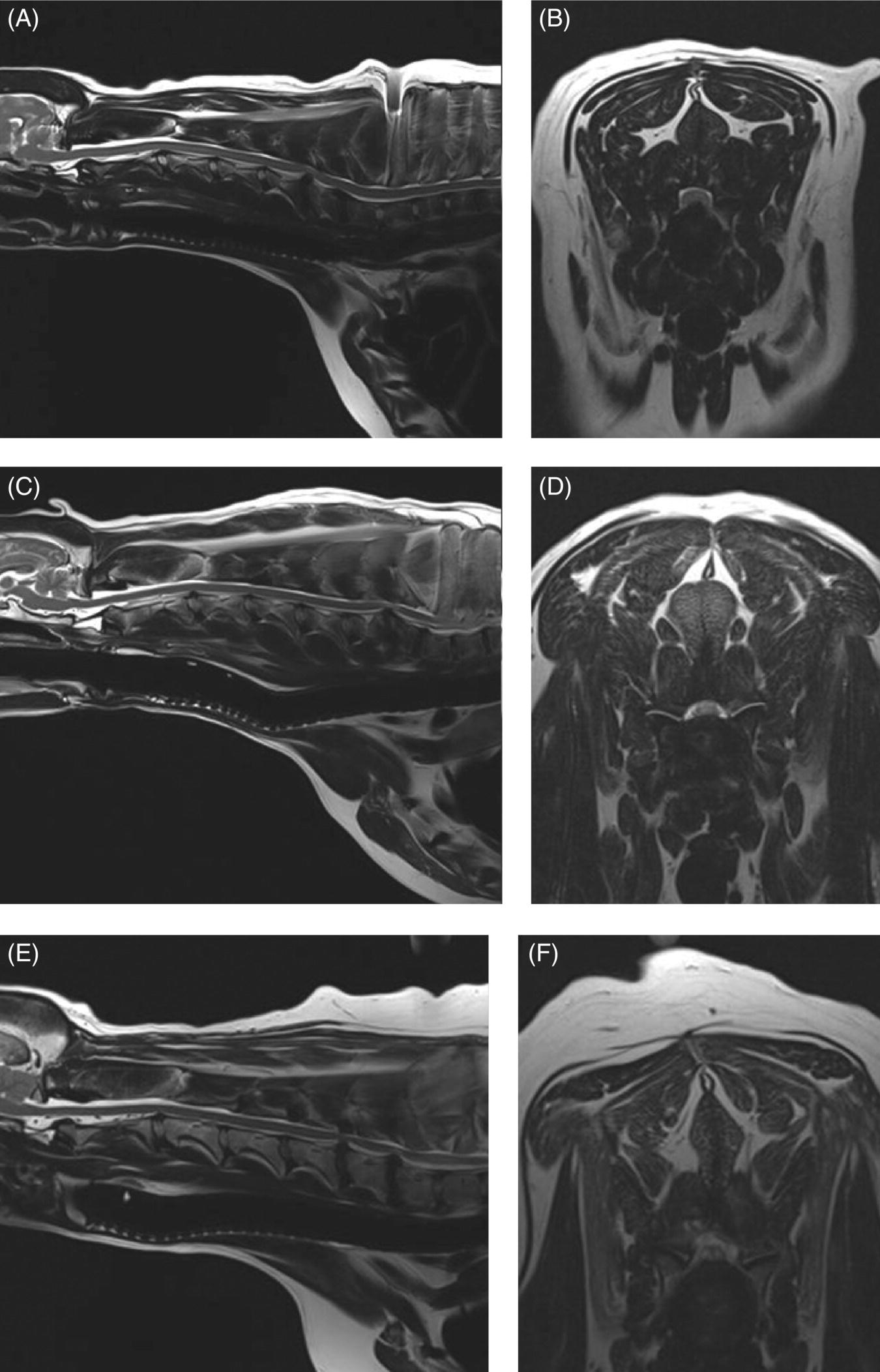
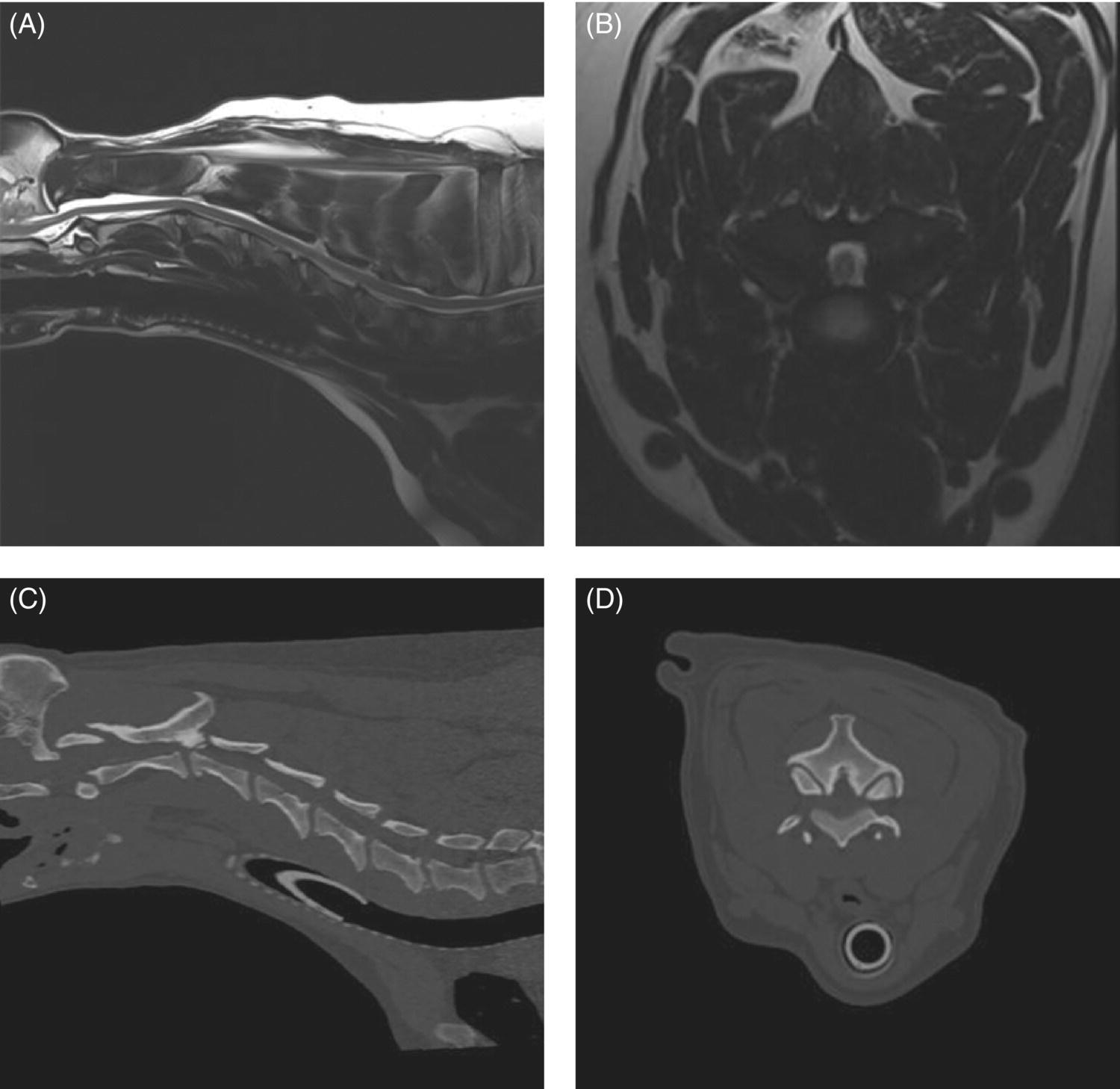
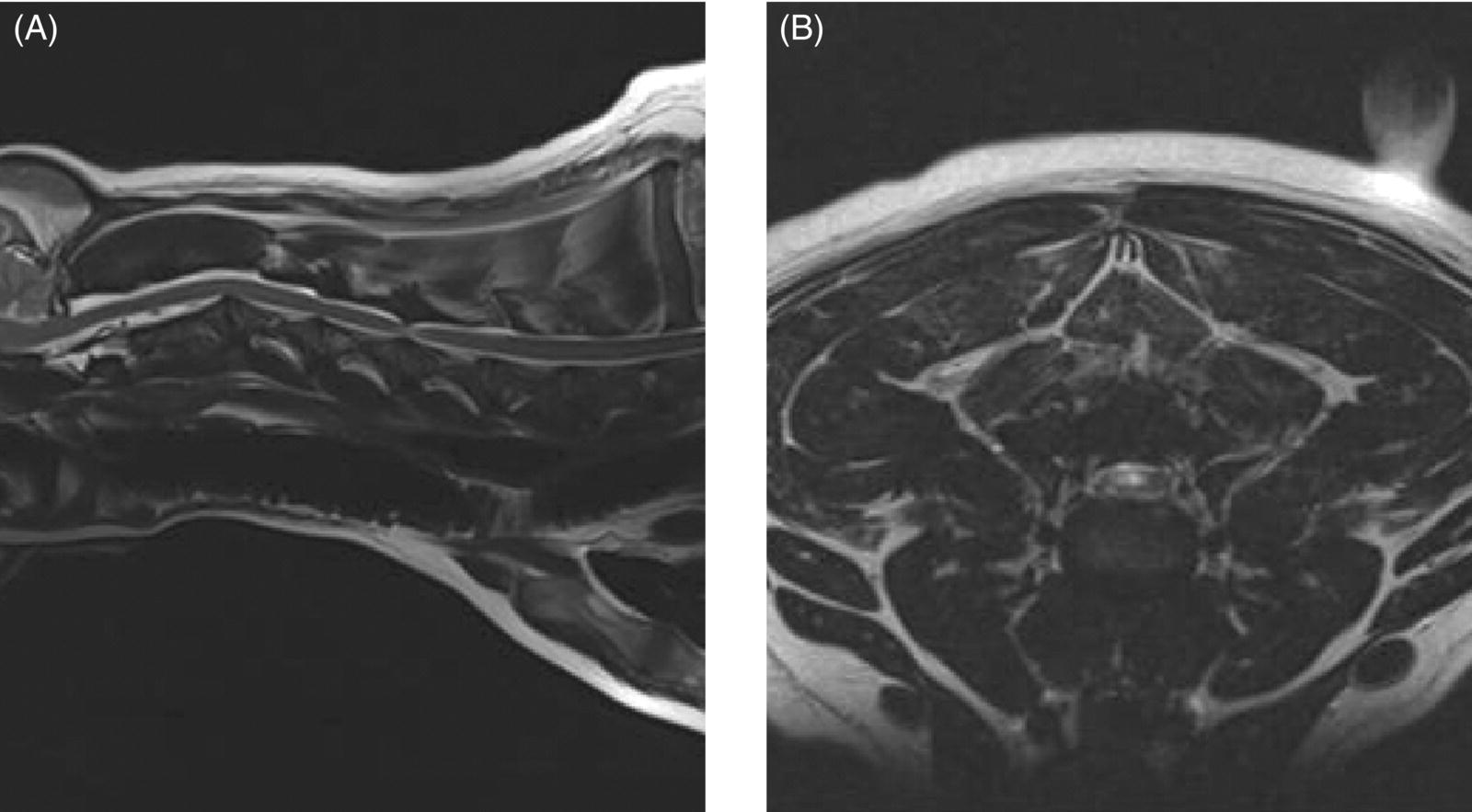
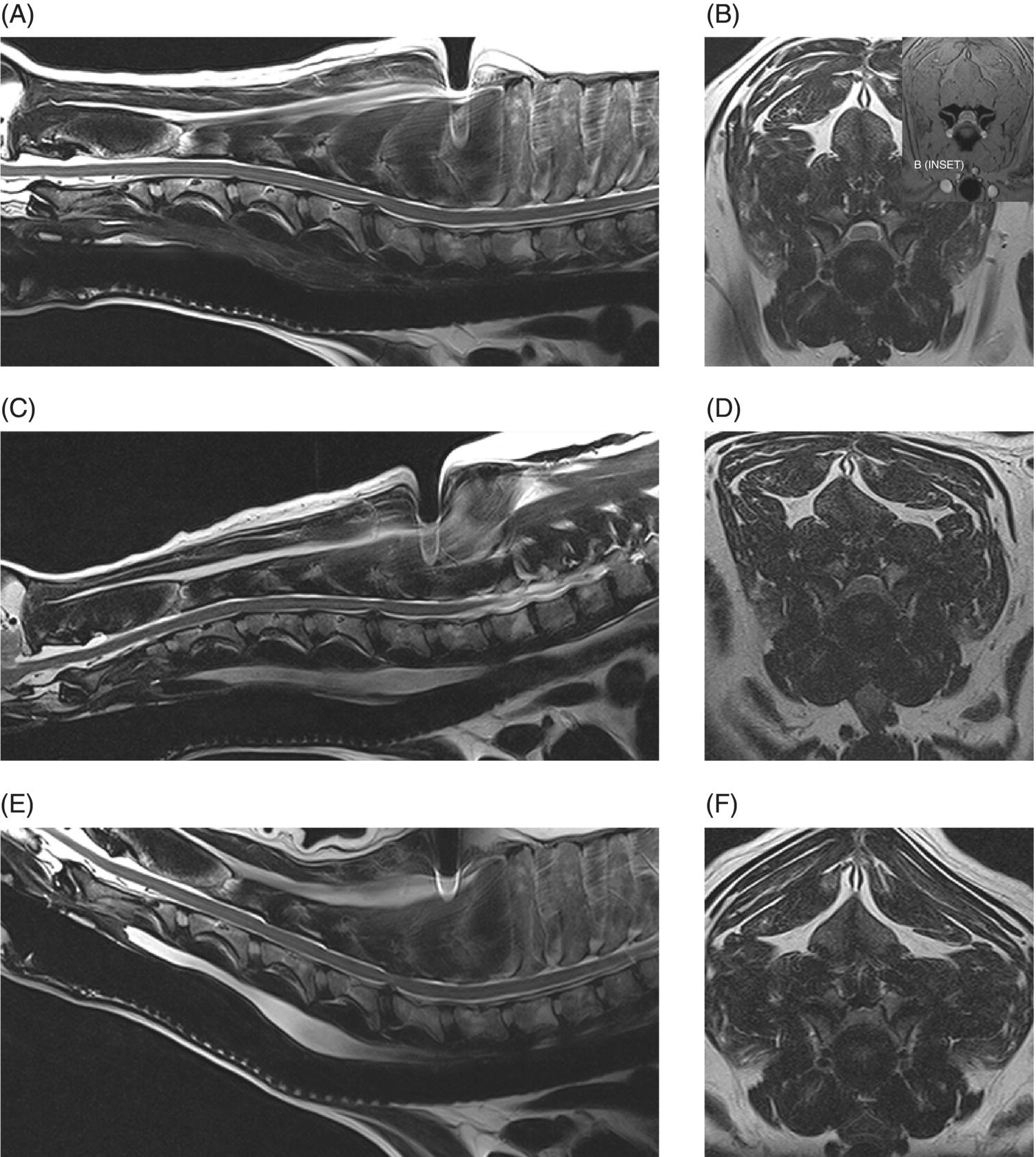
7 Noel Fitzpatrick and James M. Fingeroth The term “wobbler” disease is a rather casual, lay-type description of signs associated with mid-to-caudal cervical spinal cord disease seen in large and giant-breed dogs. There are classically two major forms of the syndrome, one most commonly seen in young giant-breed dogs (great Danes being the most representative breed) and the other more common in older large-breed dogs (Doberman pinschers, most typically). Whether these are part of a spectrum of a single disease entity or whether they are completely different diseases that share a common location and resultant neurologic signs remains controversial. When considered as a single syndrome, the term “wobbler” disease is the most commonly used synonym for what is more properly termed cervical spondylomyelopathy (CSM) [1]. CSM is a multifactorial neurological disorder characterized by abnormal development of the cervical spinal column. Typical lesions include vertebral canal stenosis, disc protrusion, ligamentous hypertrophy, vertebral column subluxation, and articular process hypertrophy leading to varying degrees of neurologic deficits and neck pain [1–4]. Clinical signs are associated with spinal cord and nerve root compression in addition to facet inflammation and paraspinal muscle spasm. Compressive lesions may be dynamic (improving with flexion or extension) or traction responsive (improving after application of linear distractive force), or they may be static (remaining unchanged by positional alteration) [5, 6]. Figure 7.1 depicts three different types of static compression in Doberman dogs attributable to disc protrusion and/or vertebral malformation/subluxation/“tipping” in disc-associated wobbler syndrome (DAWS). Figures 7.2 and 7.3 depict static compression in a great Dane and a basset hound affected by osseous proliferation narrowing the spinal canal and producing stenotic myelopathy or osseous-associated wobbler syndrome (OAWS). Figure 7.4 depicts the effect of flexion and extension on a Doberman affected by DAWS showing how the compressive lesion is dynamically compressing the spinal cord. Figure 7.1 T2-weighted MRI scans in midsagittal and transverse planes from different Doberman dogs illustrating three different patterns of disc-associated wobbler syndrome (DAWS). (A and B) A nine-year-old Doberman with intervertebral disc degeneration evident at C5–C6 and C6–C7. Central disc protrusion encroaching on the dural tube and contained spinal cord is evident on the transverse section at C5–C6. (C and D) A five-year-old Doberman with multiple sites of cervical intervertebral disc degeneration. The C6–C7 disc has undergone protrusion in association with deformity of the cranial end plate of C7 and dorsal “tipping” of the C7 vertebral body. On the transverse section, high signal intensity is evident in the spinal cord parenchyma. (E and F) A ten-year-old Doberman with multiple sites of cervical intervertebral disc degeneration. Disc protrusion is notable at C5–C6 and C6–C7, and a transverse section at C5–C6 shows high signal intensity within the spinal cord indicating compression-associated pathology. Figure 7.2 A four-and-a-half-year-old great dane with bony compression of the cervical dural tube and spinal cord, an example of osseous-associated wobbler syndrome (OAWS) or stenotic myelopathy. (A and B) T2-weighted midsagittal plane image does not accurately define the spinal cord-compressing elements, while the transverse section at C3–C4 levels clearly demonstrates abaxial encroachment of the vertebral canal by enlarged and malformed vertebral facets. (C and D) CT scans in parasagittal image plane 2 mm lateral to midsagittal demonstrates osseous proliferation of the dorsocaudal aspect of C2 vertebral lamina. This is further manifested in the transverse plane image at this level, where narrowing of the vertebral canal is clearly apparent. Figure 7.3 T2-weighted MRI scans in midsagittal (A) and transverse (B) planes of a 5-year-old basset hound manifesting extradural compression of the spinal cord at C3–C4 and C4–C5. Dorsal and ventral compression (“hourglass compression”) is evident at C4–C5, and high signal intensity within the spinal cord parenchyma is indicative of compression-associated pathology. Spinal cord compression is caused by a combination of disc and osseous-associated elements. Figure 7.4 T2-weighted MRI scans in midsagittal and transverse planes of an 8-year-old Doberman dog affected by degenerative disc protrusion at C5–C6, demonstrating the effect of flexion and extension to illustrate the dynamic effect of the extradural compression. (A and B) are neutral positioned; (C and D) show the effect of flexion, which produces more compression of the cord than neutral; (E and F) show the effect of extension, which produces even more compression than flexion. (B (inset)) is a T2* gradient-echo transverse image of the same patient at the same level as the T2-weighted image yielding superior definition of the osseous boundaries. CSM is likely the most common disease of the cervical spine of large- and giant-breed dogs. Doberman pinschers are at increased risk (incidence rate approximately 60%) and are usually presented when middle aged [7]. Other breeds, including great danes, are also commonly affected, and this breed most commonly presents when young [2, 6, 8–10]. The C5–C6 and C6–C7 vertebral levels are most frequently affected, hence the common autonym of caudal cervical spondylomyelopathy (CCSM). Lesions affecting both of these levels simultaneously have been recognized in approximately 20% of affected dogs [11]. The question whether wobbler disease is or is not related to disc disease is probably patient dependent. Disc disease when present, often occurs concomitant with multiple other manifestations of osseous and positional anomalies of vertebrae in affected dogs, but cause or effect has been difficult to establish. This is a complex disease syndrome where different morphologic changes can cause various clinical signs. There is poor correlation between either the type or severity of the disease as diagnosed on imaging modalities and the magnitude or duration of clinical signs [12]. The disease may progress for prolonged periods and clinical debilitation can be triggered by relatively minor trauma. This is particularly true of disc-associated CSM or DAWS, since motion can exacerbate disc protrusion resulting in acute deterioration [4, 13, 14] (Figure 7.3). Moreover, such deterioration may be difficult to address because of the chronicity of preexisting spinal cord abnormalities [4] (Figure 7.1). Whether wobbler disease is related to disc disease has practical and prognostic relevance for a particular patient and for a population of affected dogs. When a dog is classified as being affected by DAWS based on appropriate imaging, there are significant implications regarding treatment. The fact that more than 20 different surgical techniques have been reported for treatment of CSM implies that no single technique is able to completely address all of the variable manifestations of this complex disease. With appropriate classification however, more refined protocols for intervention are possible, which might in turn improve surgical outcomes. The etiology of CSM is unknown. Genetic, congenital, conformational, and nutritional causes have been proposed [4]. Though canine CSM shares similarities with cervical spondylotic myelopathy of humans, etiology in human patients is likely different from dogs [15]. It has been suggested that vertebral instability may predispose to chronic degenerative disc disease in dogs, or that disc disease may itself be a primary entity and may distort the dorsal longitudinal ligament, exacerbating cord compression [1, 13, 16]. However, as discussed in the following, the whole issue of “cervical vertebral instability” (CVI) remains very controversial and undetermined. The first description of the syndrome in basset hounds suggested that the disease was likely to be sex-linked inherited [17]. A study in Borzois suggested that CSM was inherited with an autosomal recessive pattern, but no explanation was given why only females were affected [9]. In spite of the frequency of the disease in Dobermans, previous studies have failed to demonstrate the mode of inheritance implied by the high prevalence in the breed [18–21]. Computed tomography of the cervical spine of neonatal Dobermans revealed stenosis of the cranial vertebral canal and asymmetry of the vertebral bodies of the fifth, sixth, and seventh cervical vertebrae, with the seventh most affected, intimating that Dobermans may be born with congenital vertebral canal stenosis [21]. Regarding body conformation, it had been proposed that abnormal forces exerted by a large head on a long neck along with a rapid growth rate may lead to abnormal stresses between vertebral bodies, inciting vertebral changes and spinal cord compression [22]. However, no correlation was found between radiographic changes associated with CSM in 138 Dobermans aged 1–13 years and head size, neck length, body length, or height at the withers [21]. Overfeeding and excessive dietary calcium were shown to increase the incidence of CSM in great danes [23, 24], but CSM prevalence has not decreased in spite of abandonment of such feeding regimes. In a study of Dobermans affected by CSM, no association was found with dietary factors [21]. Whether CSM is related to disc disease or not, osseous malformation or soft tissue proliferation has particular relevance in terms of whether lesions compressing the spinal cord or nerve roots are static or vary with neck position [25]. This may prompt consideration of surgical interventions seeking either to preserve motion or to remove motion. Pathogenesis of CSM includes primary developmental anomalies and secondary degenerative changes leading to vertebral canal stenosis and spinal cord compression [13]. Degenerative disc disease has been implicated in large-breed dogs such as the Doberman pinscher, where CSM is often both dynamic (influenced by position) and traction responsive (influenced by distractive force). Concurrent hypertrophy of the dorsal annulus fibrosus, hypertrophy of the dorsal longitudinal ligament, or concomitant osseous anomalies can exacerbate spinal cord compression, either symmetrically or asymmetrically, most commonly at the C5–C6 and/or C6–C7 intervertebral disc (IVD) spaces [1, 25, 26]. Thus, DAWS may represent dual pathogeneses of intervertebral disc disease and osseous malformation predisposing to changes in soft tissue morphology or facet hypertrophy. Both disease pathways likely have a combined effect producing dynamic and static spinal cord and nerve root compression. DAWS as the primary manifestation of CSM has been reported as high as 96% in Dobermans in one study [27] and 82% in large-breed dogs in another study [28]. DAWS appears to be distinct from the syndrome of CSM that occurs more commonly in giant breeds such as the great dane, where static osseous compression attributable to dorsoventral or mediolateral vertebral canal narrowing associated with misshaped vertebral arches, articular facets, or pedicles, and secondary arthritic proliferative change of the facets with or without vertebral misalignment often constitutes the primary disease mechanism [29]. In this breed, whether osseous change is congenital or secondary to abnormal mechanics remains speculative, but it is clear that OAWS is etiopathogenically distinct from DAWS [8] (Figures 7.1 and 7.2). Extradural synovial cysts may also be present secondary to degenerative arthritic facet changes, leading to axial compression [30, 31]. Osseous changes were the primary cause of compression in 77% of the giant-breed dogs affected by CSM in one study [28]. The marked difference in both lesions and typical age of onset once again inspires the question of whether DAWS in Dobermans and OAWS in great danes represent two different disease processes that have been improperly lumped into a single disease syndrome. The most common sites of compression are at C5–C6 and C6–C7 in both large-breed (91%) and giant-breed (72%) dogs [28]. Multiple sites of compression have been documented in 52% of large-breed dogs and 86% of giant-breed dogs [28]. The phenomenon of OAWS in giant breeds produces severe, absolute vertebral canal stenosis [28]. Disc protrusion and ligamentous compression may also contribute to the manifestation of clinical signs, but in these dogs, disc-associated pathology is generally secondary [32] (Figure 7.2). Stenosis, instability, and disc morphology may all contribute to the pathogenesis and the manifestation of clinical signs associated with CSM. The relative impact of each contributor is the subject of ongoing investigation and whether disc disease is a significant factor is an important focus for further research. CSM-affected Dobermans have relative stenosis throughout their cervical vertebral canal compared to clinically normal Dobermans [33] (Figure 7.2). This suggests that that intrinsic conformational disturbance may predispose affected animals to clinical signs when concomitant encroachment occurs for any other reason, such as intervertebral disc protrusion [33, 34] (Figure 7.3). Corroborative evidence from human patients has documented that a small vertebral canal contributes to the development of cervical spondylotic myelopathy and the diameter of the spinal cord has been shown to correlate with clinical signs of myelopathy, duration of disease, and speed of postsurgical recovery [34–40]. It is important to acknowledge that 25–30% of clinically normal Dobermans have some degree of spinal cord compression on magnetic resonance imaging (MRI), 75% have disc degeneration, 100% have some degree of disc protrusion, and 68% have foraminal stenosis [33, 41]. However, rather than drawing into question the proposed pathogenic mechanisms of CSM, this likely represents variance in terms of how well an individual dog tolerates spinal cord or nerve root compression [14]. Also, a dog that may be clinically normal at the time of assessment may yet be at risk for the later development of signs; DAWS in Dobermans is notorious for having a late onset of signs, with many dogs not affected until past 7 years of age [14, 42]. Functional impairment due to spinal cord compression is a common manifestation of DAWS in large-breed dogs, but in our experience, large-breed dogs may also present with pain alone or with unilateral thoracic limb lameness. These may be attributable to nerve root signature secondary to foraminal impingement. As in humans, the presence in dogs of disc degeneration or foraminal stenosis per se, whether dynamic (intermittent claudication) or static, does not necessarily correlate with clinical signs [34, 43, 44]. But in our experience, this can produce persistent or intermittent clinical signs in some individuals. In affected humans, foraminal stenosis is a key contributor to pain associated with cervical spondylotic myelopathy, whether due to articular facet proliferation, lateralized disc herniation, or osteophyte formation [16, 45, 46]. Another factor that may account for a discrepancy between the degree of compression and clinical signs is that in giant-breed dogs where OAWS may be present from a very young age, the lesions may become symptomatic over a longer period, even with a lesser degree of compression [28]. The ratio of spinal cord diameter to vertebral canal diameter may change as the animal matures, and consequently signs may develop as the spinal cord effectively “outgrows” the surrounding vertebral canal during late juvenile development. Additionally, not only can vertebral canals be more “funnel shaped” cranially in such dogs, but intervertebral foramina are relatively larger in small breeds than in large and giant breeds [47], where the cranial aspect of the foramen is dorsoventrally flattened and the caudal region of the foramen is bilaterally narrowed (Figure 7.3). Therefore, clinical signs of stenosis are potentially triggered by a relatively smaller volume of additional soft tissue or osseous encroachment in giant breeds than in smaller breeds. This is especially so in the caudal cervical spine where the relative cross-sectional area of the spinal cord is larger [33]. Instability has been defined as a loss of spinal motion segment stiffness, such that physiologic force produces disruption of intervertebral relationships, inducing initial or subsequent damage to the spinal cord or nerve roots with incapacitating deformity or pain [15, 48, 49]. This is very distinct from and should not be considered synonymous with dynamic CSM lesions, which worsen or improve with different positions of the cervical spine [36, 50] or physiologic hypermobility, where increased segmental mobility is reversible and does not induce disc or facet pathology [49]. The canine cervical spine has more inherent mobility than the human cervical spine [51, 52], and dynamic compression may be a greater contributor to CSM than putative intrinsic instability [14, 51, 52]. In fact, restricted rather than excessive motion may produce clinical signs in some scenarios. For example, osteophyte formation subsequent to disc degeneration may contribute to increased stiffness of a vertebral motion unit and may also produce foraminal impingement and pain [53]. Nevertheless, there is evidence that both static and dynamic factors contribute to CSM regardless of the cause or direction of compressive lesions [32]. Spinal cord stretching induced by repetitive flexion and extension over a space-occupying lesion, such as a protruded intervertebral disc, would likely augment the effect of compression over time and produce strain and stress within the spinal cord, both of which are considered key mechanisms of spinal cord injury in cervical spondylotic myelopathy in humans [54–56] (Figures 7.1 and 7.3). Whether spinal vertebral segments in affected patients are unstable or physiologically hypermobile is controversial [57], but instability has long been implicated in the pathogenesis of CSM [1, 8, 18, 58]. MRI of clinically affected and unaffected Dobermans with similar mobility patterns suggested that vertebral canal stenosis is a primary phenomenon [21] and instability is likely a secondary phenomenon in terms of manifestation of clinical signs [33], contrary to earlier interpretations [18] inferring that instability may be a primary cause of disease. Instability was purportedly responsible for 3.5% morbidity and 2.5% mortality in one study [42] and in respect to instability in CSM, it is currently speculative whether disc disease is a primary or a secondary contributor. Disc disease does not necessarily infer instability [59]; however, there is a paucity of objective criteria to define the contribution of either instability or disc disease to CSM. Subjectively, we have observed that marked end-plate deformity and relative misalignment or “tipping” of adjacent vertebrae regardless of cervical positioning occur regularly in dogs affected by CSM, although sometimes this radiographic finding can be a positional artifact and be overinterpreted (Figure 7.1C and D). Whether this is a result of congenital deformity or progressive remodeling related to or contributing to instability is unknown at this time and further research is required comparing affected and unaffected dogs to elucidate whether instability is or is not a major contributor to the disease process. To date, there are few data regarding relative motion of cervical spinal segments in dogs. Various techniques have been used to measure kinematics of the human spine including radiography, computed tomography, electrogoniometry, radiostereometric analysis, optical motion tracking, and MRI [60–62]. In human patients with neck pain, 77% have an abnormal center of motion in at least one functional spinal unit (FSU, a pair of adjacent vertebral bodies plus the intervertebral disc) [63]. Early studies of cervical spinal motion in dogs focused on radiographic assessment only, where obfuscation of dynamic and stability issues may cloud interpretation [51, 52, 64]. The C6–C7 FSU had slightly decreased flexion/extension range of motion and increased left/right lateral bending compared to more cranial FSUs [47, 64]. Recently, a more specific in vitro study revealed greater mobility in the caudal cervical segments, particularly with regard to axial rotation (by 2.6 times), which is coupled with lateral bending [65]. This corroborated an earlier study finding a greater degree of rotation in the caudal cervical spine of large-breed dogs compared to smaller dogs [47]. Torsion is a more significant contributor to disc degeneration than axial compression [66], and this may in part explain why the C5–C6 and C6–C7 intervertebral discs are commonly affected in dogs with DAWS.
Is “Wobbler” Disease Related to Disc Disease?
Introduction
Etiology
Pathogenesis
Stenosis
Instability
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


