Chapter 1 Introduction to Veterinary Hematology
Reference Intervals
Determination of Reference Intervals
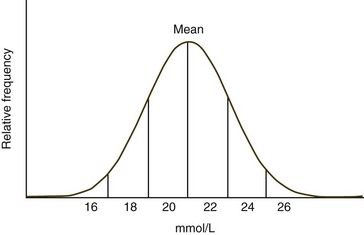
When the frequency diagram of test results from a healthy population is examined, many analytes exhibit a Gaussian or bell-shaped distribution (Fig. 1-1). When a Gaussian distribution is present, a minimum of 40 individuals (100 or more is preferred) should be assayed for statistical validity.2 In this case, the reference interval is calculated using the mean ±2 standard deviations (SD). This interval approximates the 95% confidence interval. In other words, about 95% of healthy animals have test values within this reference interval, with about 2.5% of healthy animals having values above and about 2.5% of healthy animals values below the reference interval. A common mistake made by novices is to calculate the reference interval from the mean ±1 SD. When this is done, about 32% of healthy animals will have values outside the calculated interval. If less than 40 healthy animals are available, the upper and lower values measured should be used to create an estimated reference interval.5
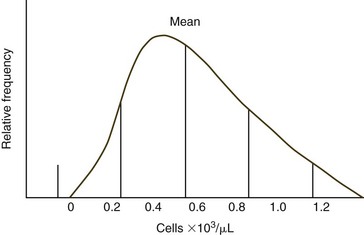
Some analytes do not exhibit a Gaussian distribution. Most commonly there is a skew toward the higher values. The use of mean ±2 SD to calculate reference intervals results in inappropriate reference intervals for skewed populations, as shown in Figure 1-2. Data may be manipulated (e.g., log or square root transformation) so that the frequency distribution of the transformed data approximates a Gaussian distribution. The boundaries are determined as before and results are retransformed to determine the reference interval. Alternatively, one can use percentiles to determine upper and lower limits, especially if large numbers of healthy animals are evaluated. Values are listed in ascending order. The lower limit is determined by the formula (n + 1) × 0.025, and the upper limit is determined by the formula (n + 1) × 0.975, where n = the number of normal animals assayed.2 If 119 animals were used, the value for the 3rd lowest animal would be used as the lower limit and the value from the 117th animal (3rd from the top) would be used as the upper limit.
Interpretation of Test Results Relative to Reference Intervals
The common usage of the 95% confidence interval to establish reference intervals means that 5% of healthy animals will be reported as abnormal for a given test. When multiple tests are done in laboratory medicine profiles, the probability of at least one test being abnormal increases with the number of tests done. For example, there is a 64% chance that at least one abnormal test result will be obtained when 20 analytes are measured from a healthy animal.6 The degree to which a test result is above or below the reference interval is generally important in deciding whether a high or low value should be taken seriously.
Sensitivity and Specificity of Tests
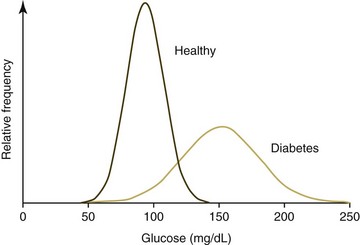
Ideally analyte values obtained from a healthy animal population would not overlap with values obtained form a diseased animal population. Unfortunately there is almost always some overlap in the distribution of individual analyte test results between the two groups (Fig. 1-3). When the disease being considered has a major impact on an analyte, little overlap in values will occur; however, extensive overlap occurs if the analyte concentration is minimally altered by the disease being considered. True positives (TPs) are positive test results from animals with the disease for which they are being tested, false positives (FPs) are positive test results for animals without the disease for which they are being tested (Fig. 1-4), true negatives (TNs) are negative test results from animals without the disease for which they are being tested, and false negatives (FNs) are negative test results from animals with the disease for which they are being tested. As can be seen in Figure 1-4, if one increases the reference interval of the healthy population in order to minimize the FPs, the number of FNs increases.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree