CHAPTER 53 Intervertebral Disk Disease
Compared with dogs, feline intervertebral disk disease (IVDD) appears to be uncommon. However, as demonstrated by numerous case reports of IVDD in cats, it should be a differential diagnostic consideration in cats with clinical signs of transverse myelopathy or radiculopathy.1–9 The veterinary literature represents feline IVDD most commonly in domesticated (Felis catus) cats; however, IVDD also has been documented in exotic, nondomesticated felids, such as Bengal (Panthera tigris tigris) and Malayan (Panthera tigris jacksoni) tigers, leopards (Panthera pardus), and the African lion (Panthera leo).10
ANATOMY OF THE INTERVERTEBRAL DISK
The intervertebral disk is a complex biochemical structure that lies between each vertebra from C2 caudally to the sacrum and coccygeal vertebrae, thus forming the amphiarthrodial joints that serve to structurally link and allow for vertebral column motion in multiple planes while resisting applied biomechanical forces.11 Each intervertebral disk consists of three anatomical components: the innermost located nucleus pulposus, an outer annulus fibrosus, and cartilaginous endplates.
The nucleus pulposus is a notochordal remnant that functions as a scaffold for development of the sclerotome into the vertebral column.11 Water is the principal biochemical component of the nucleus pulposus, and is bound within the disk matrix by proteoglycan components within the ground substance. Proteoglycan monomers of the disk are each composed of a single protein core to which numerous polysaccharide subunits, called glycosaminoglycans, are bound covalently. The annulus fibrosus is a fibrous structure that encompasses the nucleus pulposus circumferentially. Transverse sectioning of the annulus fibrosus reveals multiple lamellae of collagen fibers arranged in an eccentric fashion, such that the ventrally positioned lamellar bands are thicker and more numerous than dorsal layers. Similar to the variability reported in other species, craniocaudal disk thickness and the ratio of ventral to dorsal annular thickness in cats differs between regions of the vertebral column.12,13 In cats the contribution of the intervertebral disks to the total length of the vertebral column ranges from 12 to 18 per cent, with disk thickness being greatest in the cervical region and least in the thoracic area.12 The specific regional contributions of disk thickness to feline vertebral column length have been reported as 14 per cent in the cervical, 15 per cent in the thoracic, and 6 per cent in the lumbar regions, respectively.12 The ratio of ventral to dorsal annular thickness in cats is highest (3.2 : 1) in the lumbar area, intermediate in the cervical region (1.8 : 1), and lowest in the thoracic spine (1.3 : 1).13
The cartilaginous endplates define the cranial and caudal limits of the disk relative to adjoining vertebral epiphyses. Fibers from both the annulus and nucleus are interwoven intimately with collagenous constituents of the endplate as well as osseous elements of the adjacent vertebrae, which imparts additional strength to each disk.13 The thin, central-most aspect of the cartilaginous endplate also participates in the nutrition of the disk, allowing for diffusion of water and solutes.11
EPIDEMIOLOGY AND PATHOGENESIS
Current incidence of clinically significant IVDD in the feline population is unknown. Previous estimates of incidence in cats with clinical signs of IVDD have ranged between 0.02 and 0.12 per cent.4,7,9 In a retrospective, postmortem study of 205 cats with spinal cord disease, the prevalence rate of IVDD was 4 per cent (eight of 205 cats).3
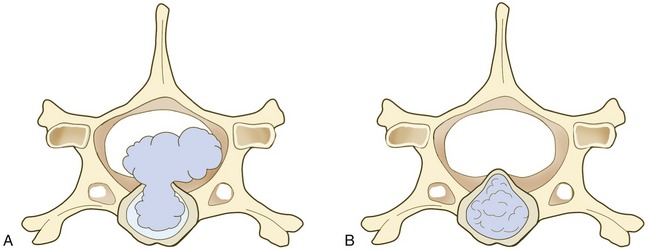
Both chondroid and fibroid disk degeneration occur in cats.4–7,14–18 Hansen type I IVDD is associated with chondroid metaplasia of the disk, and Hansen type II IVDD is associated with fibroid metaplasia, a normal aging process of the disk. Recent literature suggests that the majority of cats with clinically significant compressive myelopathy or radiculopathy have Hansen type I IVDD (Figure 53-1), characterized by herniation of nucleus pulposus through torn annular fibers with subsequent extrusion of nuclear material into the spinal canal (Figure 53-2, A).4,6–8 Published reports and clinical experience indicate that clinically significant thoracolumbar spinal cord dysfunction resulting from IVDD affects middle-aged cats predominantly, with the mean age of 7 years, although the age range (1 to 18 years) is quite variable.4 There does not appear to be a gender predilection.
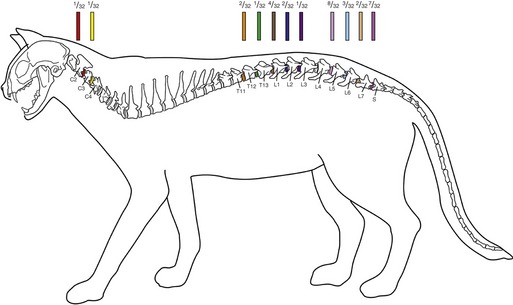
Figure 53-1 Neuroanatomical distributions and frequencies of 32 published cases of feline IVDD associated with clinical signs of neurological dysfunction from 1988-2008.* Affected intervertebral disk spaces are color-coded, with the proportion of 32 cats with IVDD at each respective site provided. Superscripts designate references compiled for source data.
(Figure prepared by Terry Lawrence, Biomedical Illustrator, Virginia-Maryland Regional College of Veterinary Medicine.)
Secondary spinal cord dysfunction can occur over any disk space in the spinal column. The caudal lumbar and lumbosacral areas are predisposed. Nearly two thirds (20 of 32) of the cases in the literature reported IVD extrusions involved the L4-L5, L5-L6, L6-L7, or L7-S1 disk interspaces (see Figure 53-1).6–9 The disk interspaces between T11-T12 and L2-L3 were involved frequently, occurring in 31 per cent (10 of 32) of clinically affected cats (see Figure 53-1).4,6,7 The proclivity for cats to jump onto objects, thereby applying increased biomechanical loads on their lumbar spines, has been suggested as a mechanism for the observed predilection for caudal lumbar disk extrusions.7,9 One study reported that the body weight of cats with L4-L5 IVDD was greater than that of cats with IVDD involving the thoracolumbar junction. This further supported the hypothesis of increased biomechanical strain on the lumbar vertebral column as a possible risk factor for IVDD of the caudal lumbar region.7
Postmortem studies suggest that degeneration of the intervertebral disk is a common incidental finding in aged cats; concurrent clinical signs of spinal cord dysfunction are rare.14–19 In these studies a significant increase in the incidence and severity of intervertebral disk degeneration was noted in cats between 11 and 14 years of age. Another study reported disk protrusions in all cats who were older than 15 years of age at the time of examination.14,15 The likelihood of multiple disk protrusions also increased with age in old cats.15 Hansen type II disk protrusions (see Figure 53-2, B) are characterized by protrusion of dorsal annular elements into the spinal canal, and account for approximately 80 per cent of disk-associated necropsy lesions reported in asymptomatic cats.14–19 This was in contrast to symptomatic cats with spinal cord dysfunction with Hansen type II disk protrusions that involved the cervical spine predominantly.17,19
Clinical signs of radiculopathy or myelopathy caused by cervical IVDD is uncommon, with a total of four cats reported.1,5,20,21 Two of these cats had acute histories associated with Hansen type I IVDD localized to the C1 to C5 spinal cord region (see Figure 53-1),1,5 and two cats had chronic Hansen type II IVDD localized to the C6 to T2 spinal cord region.20,21 Clinical signs associated with cervical IVDD may be less obvious because of the higher likelihood of Hansen type II than Hansen type I IVDD, and because of the fact that the ratio of the spinal canal diameter to spinal cord diameter is greatest in the cervical spinal canal region.1,7,17
In cats with IVDD, protrusion or extrusion of disk material into the vertebral canal is a primary event that incites concussive, contusive, or compressive types of injury processes to the spinal cord tissue. These mechanical forces initiate a cascade of vascular, biochemical, and inflammatory mediators that subsequently cause secondary tissue injury to the spinal cord. Ischemia is postulated to be a principal contributor to acute spinal cord injury by perpetuating the secondary injury processes, such as free radical generation and cellular excitotoxicity.22 Spinal cord ischemia also can be secondary to physical destruction or thrombotic obstruction of the microcirculation, functional vasospasm induced by numerous biochemical mediators associated with the secondary injury cascade, or exacerbated by systemic hypotension resulting from loss of autoregulation.22
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree