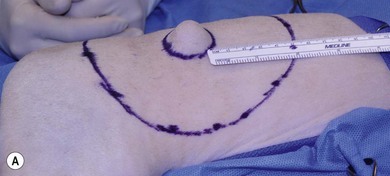
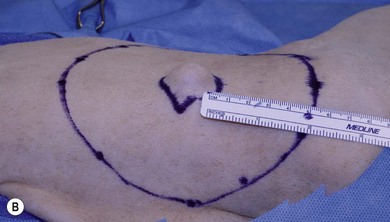
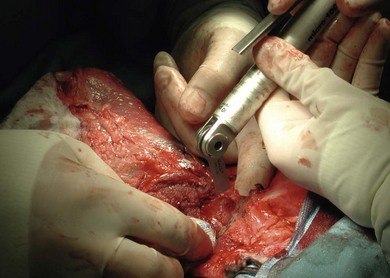
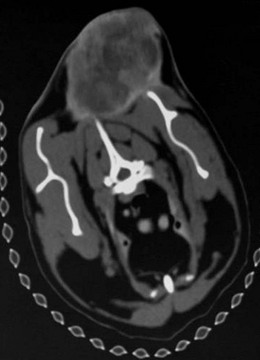
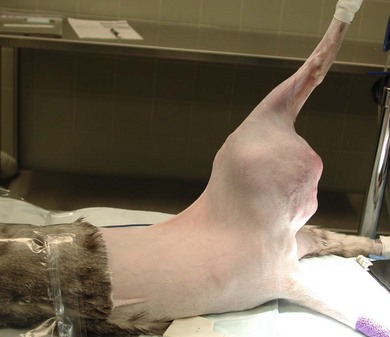
Chapter 22 Sarcomas developing at the injection sites of vaccines have been recognized in cats since the early 1990s.1,2 They can be highly invasive tumors with a tendency for local recurrence. Early aggressive multimodality treatment after advanced imaging gives the best chance for a cure. There is strong epidemiologic evidence that links the administration of inactivated feline vaccines and subsequent development of sarcomas at the site of injection. Other causes have been implicated, including sites of antibiotic and lufenuron administration.3–5 A fibrosarcoma, with features of a vaccine-associated tumor, developed at the site of a deep non-absorbable suture in a cat.6 It is now believed that vaccines are not the only agents capable of causing the development of sarcomas at the injection site, but instead virtually anything that produces local inflammation has the potential in susceptible cats.5 A leading hypothesis for the etiopathogenesis is that the inflammatory reaction leads to uncontrolled proliferation of fibroblasts and myofibroblasts that undergo malignant transformation in a subset of cats.7 Transition zones from inflammatory granuloma to sarcoma have been identified on histopathology as well as microscopic foci of sarcoma located in areas of granulomatous inflammation, which again strongly supports the idea that inflammation precedes the sarcoma development.8,9 As the incidence is relatively low and vaccines are the compounds that are given to most cats in the population with any frequency to make any correlation, vaccines have received the most attention as potential causative agents and hence most of the research has focused on vaccines.10 Strong epidemiologic evidence has demonstrated an association between the administration of inactivated feline leukemia virus (FeLV) and rabies vaccines and subsequent development of soft tissue sarcomas.2,11–13 Associations have also been reported between feline panleukopenia and feline rhinotracheitis vaccines.7,11,14,15 The true incidence of sarcoma development after vaccination is unknown. Some studies report an incidence of sarcomas per vaccines administered,13 whereas others report a prevalence of sarcoma per cat.12 The incidence is estimated to be between 1/1000 and 1/10 000 vaccines administered.7,8 One epidemiologic study determined the incidence of post-vaccination injection site sarcoma to be 0.63 sarcoma/10 000 cats vaccinated and 0.32 sarcoma/10 000 doses of all vaccines administered.16 Therefore, the incidence is now believed to be 1 case/10 000 to 30 000 cats vaccinated.16 It appears that the reaction to vaccines is additive and that the likelihood of sarcoma development increases with the number of vaccines given simultaneously at the vaccine site.13 In a retrospective study, it was determined that the risk a cat would develop a sarcoma after administration of a single vaccine in the cervical–interscapular region (a site not recommended any more by the Vaccine-Associated Feline Sarcoma Task Force and American Association of Feline Practitioners8,17) was 50% higher than the risk a cat not receiving any vaccines at this site would have. The risk for a cat given two vaccines at the same site was approximately 127% higher, and the risk for a cat given three to four vaccines was 175% higher.8,13 Time to tumor development in cats following vaccination has been reported to be between four weeks and ten years.7,16 It has been postulated that most tumors will develop within three years after vaccination.16 Tumor types that have been reported at the sites of vaccination are fibrosarcoma (most common), malignant fibrous histiocytoma, extraskeletal osteosarcoma, rhabdomyosarcoma, myofibrosarcoma, undifferentiated sarcoma, liposarcoma, and chondrosarcoma.5,18,19 Feline injection site sarcomas are usually highly aggressive, poorly circumscribed, not encapsulated, and more invasive locally than non-injection site sarcomas, making them more challenging to treat;20,21 they are also more likely to recur after surgical excision than non-injection site sarcomas.11 When compared to non-injection site sarcomas, injection site sarcomas have a higher inflammatory response, as characterized histologically by a predominately lymphocytic infiltration, along with increased necrosis and mitotic rates and the presence of multi-nucleated giant cells.20,22,23 Injection site sarcomas have a propensity for higher histologic grade than non-injection site sarcomas.23 Although in one study, more injection site sarcomas were of lower grade,24 in two other studies, high grade sarcomas (grade III) were the most common, accounting for 46% and 59%, respectivelty.21,25 A biopsy-confirmed injection site sarcoma needs to be staged. Cats with suspected injection site sarcoma based on history and location of the tumor can have the staging process started (see Chapter 14) before or after the biopsy procedure is performed but always before definitive treatment is initiated. Complete blood count, serum biochemistry panel, urinalysis, along with FeLV and feline immunodeficiency virus (FIV) tests will determine the overall health status. Although no association is apparent between viral status (FeLV, FIV) and tumor development, the course of the disease may be altered because of compromise of the immune system.7 The chest should be evaluated for evidence of metastasis, which occurs in 5–24% of cases.3,19,24,26–28 Chest radiographs can be performed using three views or alternatively computed tomography (CT) can be performed to evaluate the chest and lungs. CT is more sensitive than radiographs in detecting pulmonary metastatic lesions.29 Regional lymph nodes should be evaluated through palpation, radiographs, or ultrasonography, and cytology where applicable. Metastasis occurs primarily to the lungs but other sites such as regional lymph nodes, mediastinum, pericardium, liver, skin, and pelvis have also been reported.7,19,30–32 Advanced imaging techniques such as CT or magnetic resonance imaging (MRI) are extremely helpful, and considered by many to be mandatory in planning a surgical resection and potentially deciding to perform radiation therapy in the neoadjuvant setting (Fig. 22-1). In a study to evaluate the usefulness of CT imaging of vaccine-associated sarcomas, the CT image revealed the tumor to be, on average, twice as large as determined by physical examination and caliper measurement, and recommendations regarding the treatment were often altered based on the CT results.7,33 In another study, the use of CT resulted in the reclassification of 17/26 subcutaneous neoplasms in dogs to a more advanced clinical stage as compared to physical examination.34 Even patients that have had a previous surgical excision can benefit from advanced imaging as it can provide information regarding the extent of the previous surgical field and therefore the area that needs to be re-excised or included in the radiation treatment field.7 Figure 22-1 CT image demonstrating the relationship of the tumor with local anatomical structures. The tumor is seen extending to the soft tissue–bone interface with the scapula and spinous process of the vertebra. For a curative-intent surgery, this will necessitate removal of those structures (osteotomy of the spinous process and partial scapulectomy) to achieve an adequately deep surgical margin. Surgery is one of the most important components of the treatment regime for these tumors. However, attempts at simple excision (i.e., debulking or marginal excision) are rarely curative and ultimately lead to local recurrence with a more difficult second surgery.5,7 Therefore wide to radical excision is the recommended surgical approach. Multimodal treatment consists of a combination of surgery, radiation and/or chemotherapy, and a multimodal treatment to include irradiation is considered the standard of care to treat injection site sarcomas.5 However, results of studies evaluating radical surgical excision alone question the necessity and the benefit of radiation therapy with this type of surgery. As with any tumor, vaccine-associated sarcomas should be treated early when small. Unfortunately, these tumors can be very fast growing and the cat is often presented when the tumor is relatively large (Fig. 22-2). Given that these tumors grow in the subcutaneous areas (where natural barriers to tumor growth are absent), there are no definitive criteria that define a resectable versus a non-resectable tumor. The locally invasive nature of these tumors necessitates wide to radical excision to achieve the longest disease-free intervals (see surgical treatment and prognosis sections). These tumors do not invade bone, but the locally invasive injection site sarcomas can extend to the soft tissue–bone interface, necessitating removal of bone as a deep surgical margin with radical excision (see Fig. 22-1).21 In one study, 57% of cats that had an injection site sarcoma removal required an ostectomy of some sort.21 Figure 22-2 A cat with a large tumor on the caudal aspect of the pelvic limb. Because of the proximal extension of the tumor, a mid to caudal hemipelvectomy was performed (see Fig. 22-5). Aggressive surgeries are generally well tolerated by cats.21,24 With the advancement of reconstructive surgical techniques and the availability of polypropylene mesh, in conjunction with advancements made in critical care, extensive body wall resections are possible. When performing a thoracic wall resection as many as eight ribs can be successfully removed (Charles Kuntz, personal communication). Cats can survive massive resections, even following tumor removal resulting in a wound of over 15 cm in diameter.21 Therefore relatively large tumors can still be amenable to wide or radical surgical excision and the size of the tumor is rarely a limiting factor in the decision to perform surgery. For the purpose of feline injection site sarcomas, wide excision is the removal of the tumor with 2–3 cm of normal tissue beyond the edges of the tumor and one fascial plane (one muscle) deep to the tumor, whereas radical excision is the removal of the tumor with 5 cm of normal tissue beyond the edges of the tumor and two fascial planes (two muscles) deep to the tumor, including bones that are part of these margins.21 The rationale to remove muscles, which are covered by fascia, deep to the tumor is that fascia is a collagen-rich, vascular-poor tissue that has been shown to be a natural barrier against cancer and it is more difficult for tumors to grow across these collagen-rich, vascular-poor tissues.35 Sometimes it is possible to remove the fascial layer only without having to remove the muscle. This is possible when the fascia peels easily away from the muscle it covers. But in most instances, the fascia is too intimate to be peeled from the muscle and it is best to remove the entire muscle thickness. Because of the variation in anatomic location and tumor size, the technical details of the surgical excision will vary from cat to cat (Box 22-1). Adherence to sound principles of surgical oncology is important throughout the surgical procedure (see Chapter 14). If the tumor comes close to a bone such as a dorsal spinous process or scapula (see Fig. 22-1), these bony structures are excised en bloc with the tumor. Therefore osteotomies are performed as part of the tumor excision as opposed to removing the tumor by peeling off the soft tissues from the bone and then performing the osteotomies. Even though a tumor might not be in contact with a bony structure, osteotomies may still be required to achieve appropriate and adequate deep margins. This is particularly applicable for tumors that cross the dorsal midline in areas where there are no single fascial/muscle planes. Therefore for these tumors, it is best to remove the dorsal spinous processes of the vertebrae in the surgical field of resection. Osteotomies of the dorsal spinous processes can be performed with Liston bone cutting forceps, power burr, power oscillating saw, or gigli wire saw; the latter option is not usually recommended. The best excision of any malignant tumor is one where entry into the tumor or its capsule is avoided. If the tumor, or its capsule, is visualized or entered during the dissection there is the risk of contamination of the surgeon’s gloves and/or instruments with tumor cells resulting in potential seeding of these cells to other areas of the surgical wound bed. Tumors growing in the interscapular area can require scapulectomy. Typically, a partial scapulectomy is performed (Box 22-2). If a complete scapulectomy is deemed necessary, it is best to perform a full forelimb amputation. The reason for this is not that cats cannot do well with a complete scapulectomy, but rather because better margins are achieved with a full amputation of the limb. Partial scapulectomy involves the excision of the dorsal aspect of the bone. As a general rule, the less bone removed, the better the limb function. However, bone should not be preserved for the sake of function as this may compromise the completeness of excision of the tumor. Tumor excision with adequate margins is the priority. Excision of tumors located on the caudal aspect of the trunk, over the pelvis, may require hemipelvectomy (Box 22-3). A total hemipelvectomy is the removal of the limb and entire hemipelvis (os coxae) including the ilium, acetabular bone, and pubis and ischium to midline (Fig. 22-4A). A middle-to-caudal partial hemipelvectomy is removal of the hemipelvis including the acetabulum with amputation of the hind limb and a portion of the ischium to the midline (Fig. 22-4B). A middle-to-cranial partial hemipelvectomy includes removal of the hemipelvis from immediately caudal to the acetabulum to the sacroiliac joint combined with pelvic limb amputation (Fig. 22-4C). Caudal partial hemipelvectomy is defined as removal of the ischium with preservation of the acetabulum and limb (Fig. 22-4D). For tumors that are located more cranially, removal of the wing of the ilium alone may be sufficient, with a single osteotomy performed cranial to the sacroiliac joint.
Injection site sarcomas
Epidemiology
Clinical signs and tumor staging
Imaging
Treatment options
Decision making
Surgical excision
Osteotomies
Scapulectomy
Hemipelvectomy
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Injection site sarcomas