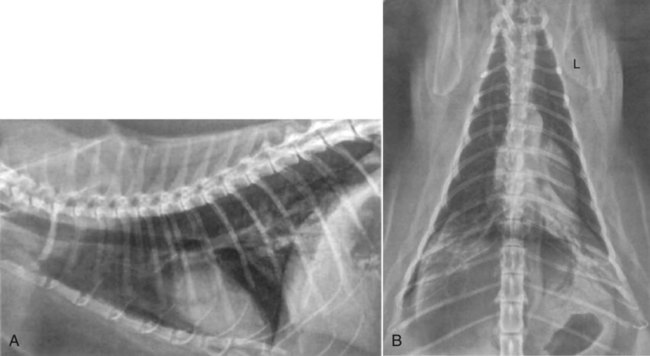
Influenza viruses are in the family Orthomyxoviridae, negative-sense, single-stranded, segmented RNA viruses that infect a variety of mammals and birds. Aquatic birds are generally their asymptomatic reservoirs. Mammals, both domestic (swine, horses, dogs, ferrets) and nondomestic (mink, aquatic), poultry, and humans inadvertently contract these strains of virus that spread among the various hosts.98 Almost every winter, two genera—types A and B—produce episodic outbreaks of an acute self-limiting febrile illness in susceptible humans. Types B and C are mainly human pathogens and are only rarely seen in animals. Type A viruses, having the widest host species range and greatest mutability, can rapidly evolve into new genetic subtypes. Dogs and cats, living in close proximity to their owners, may occasionally become infected during human influenza type A outbreaks. Furthermore, dogs or cats coming in close proximity to poultry, swine, or horses or eating uncooked meat by-products from these animals may contract these influenza viral infections. Box 23-1 lists online sources of further information on influenza virus infections of dogs and cats. Furthermore, the segmented genome of influenza viruses allows for extensive reassortment during co-infection within a single host species. This drives evolution of influenza viruses in avian species but also in mammals. For example, pigs can become simultaneously infected with both human and avian strains because the different sialic acid receptors in the respiratory epithelium allow simultaneous entry of swine, human, and avian influenza viral strains. Within the porcine host, novel influenza virus A strains can originate in a sudden and dramatic fashion, through antigenic “shift” from genetic reassortment. The genome segments of the progeny viruses may be derived from different species. Pigs are, therefore, believed to serve as a “mixing vessel” for this genetic reassortment. If genetic recombination occurs, a new human influenza virus strain may arise with the ability to spread readily from swine back to people, but with a markedly different antigenic composition from the original infecting human strain. An antigenically distinct new strain may evolve to which the world’s human population has no immunity,106 leading to a pandemic. Viruses that are naturally circulating in pig populations can occasionally also cause influenza in people who are occupationally exposed. Historically, these viruses are not transmitted efficiently among humans.101 This also applies to virulent avian influenza virus strains that can occasionally spread directly to people, causing high mortality but only limited spread. Meat from poultry or swine does not pose a risk for human infections when it is properly cooked. Infections with endemic influenza viruses are restricted to the respiratory and gastrointestinal (GI) tract. Most contemporary human infections have been associated with either influenza B viruses or the H1N1, H3N2 subtypes of influenza A. Fever, myalgia, and signs of upper or lower respiratory tract infections are the most common manifestations in people. Mortality is the result of pulmonary complications. Although the 1918 H1N1 virus was derived from the avian reservoir, it is currently not clear whether the virus needed adaptation in pigs before causing the pandemic in people or whether the virus was transmitted directly between humans and pigs without adaptation in one or the other species. The 2009 pandemic influenza A H1N1 virus is uniquely different from that previously found in pigs and humans.63 Influenza viruses are enveloped and are relatively susceptible to environmental influences. Their survival is prolonged by colder temperatures and moist conditions. Common disinfectants and warm environmental temperatures with low humidity are most effective in their inactivation. In the absence of organic material, most common disinfectants (1% sodium hypochlorite, quaternary ammonium compounds and 70% ethanol, aldehydes, and lipid solvents) inactivate the virus after 10 minutes’ contact time (see Chapter 93). At pH 7.0, chlorine concentrations typically used in drinking water are sufficient to inactivate the avian virus after times of exposure as brief as 1 minute.75 Ionizing radiation and acid (pH 2.0) are also virucidal. In the absence of disinfection, survival in moist conditions is not greater than 30 minutes at 56° C (133° F) a few hours at 55° C (132° F), several days at room temperature of 22° C (71° F), and at least a month when frozen. At room temperatures canine virus isolates survive on surfaces, clothing, or hands for 48, 24, or 12 hours, respectively.5 Experimental intranasal or intravenous infections of dogs with influenza virus A,28,50,68,92 B,68,92 and C53,54,60–62 types have provided convincing evidence that these viruses can replicate in canine hosts. However, clinical signs in infected animals either were absent or consisted of a mild conjunctivitis, serous nasal discharge, and variable fever. With respect to natural infections, serologic responses to influenza A virus have been observed in dogs during human disease outbreaks.26,52,78,100 Limited data, based on viral isolation from respiratory secretions, documents sporadic natural transmission and subclinical infection of dogs with human H3N2 influenza A viruses16,32,38,59,78 and type C viruses.53,54 There is, however, no evidence for sustained transmission chains of human influenza viruses in dogs. It may be assumed that species barriers are too tight for natural transmission to occur42 despite the fact that human influenza viruses readily replicate in Madin-Darby canine kidney (MDCK) cells. In cats, antibody detection performed in the 1970s found hemagglutination inhibition (HI) antibodies against influenza viruses of subtype H3N2 in blood of clinically healthy cats.26,64,67,68,78 No antibodies against the subtypes H1, H2, H4 through H8 were found in these animals.64 As human epidemics with influenza H3N2 viruses occurred in the countries of investigation, it might be that the cats had been exposed to this particular virus. In experimental investigations cats have been susceptible to human (H3N2), avian (H7N3), and seal (H7N7) influenza virus isolates.31,67,68,78 After infection, they developed antibodies, sometimes even shed virus, but never became sick. In some of the experiments, human influenza virus H3N2, H2N2, and influenza B virus infection could also be transmitted to uninfected cage mates. These earlier studies suggested that cats might represent yet another host for various influenza viruses in nature.31 So far under natural conditions, however, the previously incriminated viruses have been isolated from cats with or without disease signs. A similar situation might occur with the pandemic swine origin influenza virus A/H1N1/09. Before its known spread to dogs and cats, this virus was only known to infect humans, pigs, horses, and birds (Table 23-1). TABLE 23-1 Summary of Influenza A Viral Strains Infecting People and Domestic Animals aPredominantly swine-origin virus. bAvian and human strain reassortment. dEquine-origin virus, spread between dogs was suggested by viral shedding but not further evaluated. eH5N1 avian-origin virus did not, while H3N2, spread readily among experimental contact dogs. In dogs, the first detections of H1N1 have been reported from China.73 Two of 52 samples from sick dogs had positive test results for the virus. Analysis of the genetic composition revealed that the viruses in the dogs were 99% homologous to viruses in human H1N1 flu cases, confirming that the dogs were infected with the pandemic virus. In New York, a 13-year-old dog with respiratory illness associated with lethargy, coughing, anorexia, and fever was found to have radiographic evidence of pneumonia.4 The dog improved after symptomatic therapy with fluids, antibacterials, and nebulization and was discharged from the hospital after 48 hours. Tests with polymerase chain reaction (PCR) confirmed that the dog was infected with H1N1 virus. Cats have also been found to be infected with H1N1 with resultant clinical illness. In separate incidents, two 13-year-old cats from Iowa9 and Utah,107 an 8-year-old cat from California,4 and two cats in Oregon48a developed signs of a respiratory infection after family members in the household were ill. As a particular example, one of the 13-year-old cats from Iowa developed dyspnea and orthopnea within 6 days after a respiratory disease in human family members.87 The cat was afebrile and dehydrated on physical examination. Bilateral multifocal caudodorsal alveolar densities were observed with thoracic radiography (Fig. 23-1, A and B). Cytologic findings of bronchoalveolar wash consisted of foamy macrophages, nondegenerate neutrophils, and small lymphocytes. These cats all had had serum antibody reactive to the pandemic influenza virus A/H1N1/09; these were the first reports of cats infected with this particular virus. The cats and their owners have recovered from their illnesses, and there is no evidence that the cats passed the virus to any humans. PCR results targeting the H1N1 virus were positive in one cat.87 However, results in other cases are often negative, perhaps relating to the low rate and transient nature of viral shedding in heterologous hosts. In cats that have died, severe necrotizing bronchointerstitial pneumonia has been found with the virus present throughout tissues in the lower respiratory tract.48a Because the amount of H1N1 virus excreted by both dogs and cats is low, there appears to be low risk of their infecting more susceptible human hosts. Even swine infected with the A/H1N1/09 strain harbor it in their tissues for no longer than 3 days after exposure to the virus.103 An H1N1 PCR test is commercially available to detect this strain of virus in dogs or cats (IDEXX H1N1 Influenza Virus RealPCR Test, IDEXX Reference Laboratories, Westbrook, ME) (see Web Appendix 5). Because of the close association of pets with humans, concern has been raised, although unnecessarily, that dogs and cats may be important in the spread or maintenance of influenza infection in people. Many reports exist of the presence in dogs and cats of antibodies against human influenza virus strains. Influenza infection that jumps between different species is often a self-limiting spread and is not transmitted between in-contact animals. See reference links in Box 23-2 for recommendations in the control of influenza outbreaks. No evidence suggests that the virus replicates sufficiently to spread from infected pets back to humans. Because of their potential infection on a clinical or subclinical basis, dogs and cats may be useful as sentinel hosts for surveillance of influenza virus infections.17 Prevention of human infection involves minimizing direct contact with animal reservoirs such as swine and poultry. All influenza H and N subtypes can be found in varying combinations in different aquatic birds, which represent the natural reservoir of all influenza viruses (see Table 23-1). Generally, influenza virus infections in aquatic birds are of low pathogenicity and are not associated with clinical signs. Viral replication is mainly restricted to the GI tract and transmission of the infection is facilitated by the cloacal–oral route. Low-pathogenic avian influenza viruses (LPAIVs) of subtype H5 and H7 are exceptional in that they can mutate enabling systemic replication of the virus in the infected avian host. Infections with these LPAIVs are associated with diverse symptoms in waterbirds and severe clinical signs in domestic poultry. Some of these viruses also acquire a broadened host spectrum.1,98 In the contemporary period, HPAIV H5N1 has been the predominant avian strain to infect dogs and cats, and the majority of this section on avian influenza discusses this infection. However, one outbreak of respiratory infection in South Korea in dogs at three veterinary clinics and one kennel was associated with an H3N2 strain of virus that likely originated from birds.83 All but one of the dogs from the veterinary clinics died; the outcome of the kennel dogs was not reported. Infection with a closely related strain was found in Southern China.47a Clinical signs in these dogs included fever, sneezing with nasal discharge, coughing, and anorexia. The dogs had signs of severe pulmonary lesions at necropsy. Antibody testing could have been helpful in distinguishing the type of influenza virus infection; however, it was not done in this report. PCR was used for identification of the virus type. In experimentally infected dogs, H3N2 is shed for up to 6 days after the onset of illness. Unlike the highly pathogenic avian-origin H5N1 strains, which produce respiratory and systemic illness; the avian-lineage H3N2 isolate, which had adapted to spread between dogs, only produced respiratory tract infection in experimentally inoculated dogs.34b Pandemic HPAIV of subtype H5N1 originated in China in 1996 and spread until 2003 to other countries in Southeast Asia. The viruses, continuously shaped by several reassortment events and by antigenic drift, established endemic infections, driven by subclinically infected duck populations, in this area. An incursion of HPAIV H5N1 of the phylogenetic cluster 2.2 (“Qinghai lineage”) into the wild bird population of northwestern China in 2005 marked the beginning of an unprecedented virus spread to other Asian regions, Europe, and Africa within a single year. Apart from causing severe clinical signs in gallinaceous bird populations, HPAIV H5N1 also exhibited a potential to infect mammals including humans. Next to humans, naturally infected mammal species were members of the order Carnivora (Canidae, Felidae, Mustelidae, and Viverridae).13,40,77,91 With regard to HPAIV H5N1 in dogs and cats, a fatal infection of a dog after scavenging on HPAIV H5N1-infected chicken carcasses was reported in Thailand.86 Several outbreaks of infection with HPAIV H5N1 have been reported to date in felids under natural conditions. The first outbreak was seen in 2003, when two tigers and two leopards suffering from high fever and respiratory distress died in a zoo in Suphanburi, Thailand.37 Shortly after, a clouded leopard died in a zoo in Chonburi, Thailand, from infection with influenza H5N1. One month later, a tiger at the same zoo was found to be infected but recovered from the disease.25 During an outbreak in a tiger zoo in Sriracha, Thailand, a total of 147 tigers died or were euthanized.89 The first evidence that domestic cats are at risk was in 2004, when 3 domestic cats from a household in Thailand, in which 14 cats had died, were tested positive for influenza H5N1.25 The virus was also detected in a domestic cat in Thailand that had died showing high fever, dyspnea, convulsions, and ataxia.85 Experimental inoculation of dogs with HPAIV H5N1 results in severe clinical illness indicating their high degree of susceptibility.16a Experimental infections using HPAIV H5N1 confirmed that cats can develop severe clinical signs after infection44,99 or after feeding on infected chicken.44 The first cases of HPAIV H5N1 infection in domestic cats in Europe were detected during the outbreak of avian influenza on the German Isle of Rügen in February 2006, during which three free-roaming cats found dead were harboring the virus.40 At approximately the same time, three cats that did not show clinical signs tested positive by PCR for influenza H5N1 in an animal shelter in Graz, Austria, after an infected swan had been brought to the shelter.47
Influenza Virus Infections
Human Influenza (H3N2 and H1N1) Virus Infections
Historical Considerations in Dogs
Historical Considerations in Cats
Influenza H1N1 Infection 2009
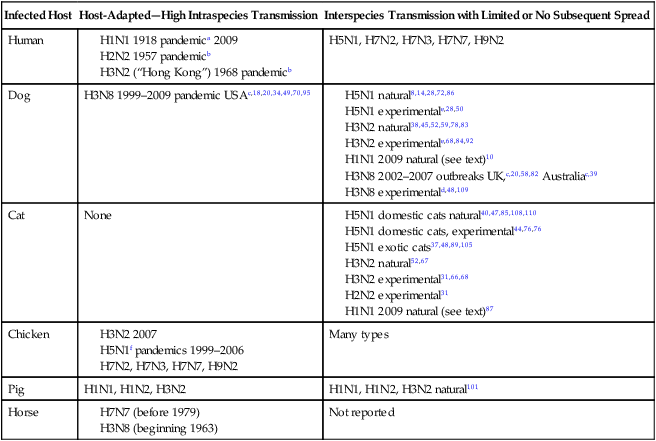
Infected Host
Host-Adapted—High Intraspecies Transmission
Interspecies Transmission with Limited or No Subsequent Spread
Human
H5N1, H7N2, H7N3, H7N7, H9N2
Dog
H3N8 1999–2009 pandemic USAc,18,20,34,49,70,95
Cat
None
Chicken
Many types
Pig
H1N1, H1N2, H3N2
H1N1, H1N2, H3N2 natural101
Horse
Not reported
Public Health Considerations
Avian Influenza (H3N2 and H5N1) Virus Infections
Etiology
Influenza H3N2 Infection
Influenza H5N1 Infection
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Veterian Key
Fastest Veterinary Medicine Insight Engine
