John Schumacher
Infiltrative Bowel Diseases of the Horse
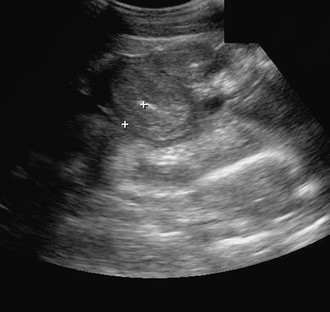
Infiltration of the horse’s intestinal tract with inflammatory or neoplastic cells may result in clinical and clinicopathologic signs of disease that are similar, regardless of the type of cell involved. Infiltration of the mucosa and submucosa of the gastrointestinal tract with large numbers of eosinophils, lymphocytes, macrophages, plasma cells, or basophils is termed infiltrative bowel disease (IBD). In some horses, these changes lead to intestinal mural thickening that can be appreciated sonographically (Figure 72-1). In other horses, intestinal thickening is not obvious on ultrasound or grossly (during surgery), and lesions are found only during microscopic examination of affected tissue. For many of the IBDs of the horse, a granulomatous reaction is a typical histologic finding. A granuloma is a tightly organized collection of macrophages (histiocytes) that congregate to eliminate or wall off a foreign substance. Regardless of the type of cell involved, horses with an IBD often have protein-losing enteropathy and malabsorption of nutrients, resulting in clinical signs that include weight loss, lethargy, diarrhea, and dependent edema. For some horses, the invading cells are inflammatory and the invasion is determined to be caused by cyathostomosis, pythiosis, fungal (histoplasmosis) or mycobacterial infection, or a potentially toxic plant (e.g., hairy vetch). For other horses, the infiltrating cells may be neoplastic rather than inflammatory. Because in many instances the cause of the bowel inflammation is undetermined, affected horses often receive a diagnosis of chronic, idiopathic, inflammatory bowel disease (CIBD). Types of CIBD reported to affect the horse include granulomatous enteritis (GE); lymphocytic-plasmacytic enterocolitis (LPE); multisystemic eosinophilic epitheliotropic disease (MEED); idiopathic, focal, eosinophilic enterocolitis (IFEE); and sarcoidosis. Proliferative enteropathy in juvenile horses caused by Lawsonia intracellularis is considered by some authors to be an equine inflammatory bowel disease, but thickened intestine in affected horses is caused by hyperplasia of enterocytes in the intestinal mucosa rather than by infiltration of inflammatory cells.
Although the horse’s signalment, clinical signs, results of abdominal ultrasound, clinicopathologic findings, and gross lesions often provide clues for diagnosis of IBD, definitive diagnosis is made on the basis of results of histologic examination of an intestinal biopsy specimen. The histologic diagnosis of CIBD is often subjective because it is based on assessment of the severity of infiltration of inflammatory cells, which is itself subjective and difficult to assess because normal populations of intestinal inflammatory cells vary in number among species, individuals, and segments of intestine. The significance of finding eosinophils or lymphocytes in the intestinal wall is especially difficult to interpret because increased numbers of these cells can be found in the intestinal wall of clinically normal horses. Tissue for antemortem histologic examination is usually obtained by celiotomy or rectal biopsy. Laparoscopic techniques, extracorporeal or intracorporeal, for full-thickness intestinal biopsy have been used successfully to collect intestinal tissue from horses with IBD. Histologic examination of a rectal biopsy specimen was useful in diagnosing GE or MEED in about one-half of horses with either of these conditions in one study, but is unlikely to be helpful in definitively diagnosing LPE or IFEE. Gastroduodenoscopy-assisted biopsy of the small intestine might aid in diagnosis of IBD if inflammatory cells have invaded the duodenum. Data concerning the normal population of inflammatory cells in the region of duodenum have been reported (see Divers et al, 2006, in Suggested Readings).
Chronic Inflammatory Bowel Diseases
Granulomatous Enteritis
Horses with GE often have grossly thickened intestine walls and adhesions of small intestine to small intestine. The ileum is most commonly and severely affected, but a large portion of the small intestine is abnormal in most affected horses; large intestine, if involved, is less affected. The lamina propria and submucosa contain foci of fibroblasts, macrophages, multinucleate giant cells, lymphocytes, and plasma cells. Granulomatous enteritis has been compared with Johne’s disease in cattle and Crohn’s disease in humans because the histologic lesions are similar. Granulomatous enteritis has been reported in multiple breeds of horses, but many reports involve Standardbreds, some of which were related, indicating a possible genetic predisposition to development of the disease. Affected horses are usually young and are almost invariably presented for veterinary examination because of weight loss and anorexia. Skin disease in horses with MEED is a feature that is often considered to distinguish MEED from GE, but some horses with GE also have skin lesions, usually involving the head and limbs, especially the coronet.
Horses with protein-losing enteropathy experience enteric loss of proteins of all molecular weights, but because globulins tend to be produced faster than albumin, the most striking clinicopathologic feature of CIBD in the horse is hypoalbuminemia. Most horses with GE are anemic, but anemia may not be evident because of a diminished plasma volume caused by low concentration of plasma albumin. Plasma transfusion may cause a marked drop in the packed cell volume of horses with GE.
Carbohydrate absorption tests of horses with GE usually reveal abnormal absorption of glucose or xylose. Malabsorption of carbohydrates is attributed to severe villous atrophy diffusely throughout the small intestine. Some horses with GE have normal carbohydrate absorption test results, presumably because the disease has not progressed to a point at which villous absorptive capacity is exceeded or because intestinal lesions are so localized that they do not interfere with absorption.
Horses with GE do not respond to treatment with the drugs used to treat Crohn’s disease in humans, such as salicylazosulfapyridine and methylsulfapyridine. A few horses with GE were reported to respond favorably to treatment with dexamethasone, but long-term survival associated with any medical treatment has not been reported. In one report, two horses with GE responded favorably to removal of several meters of grossly affected ileum and distal jejunum. Surgical treatment of most horses with GE is unlikely to be beneficial, however, because grossly evident intestinal lesions are usually diffuse and involve long segments of intestine.
The cause of GE was not determined in most reported cases despite the veterinarian having a detailed history, the use of tissue cultures, and histologic examination of affected tissues, including with electron microscopy. Aluminum toxicosis, compounded by parasitism, was diagnosed as the cause of clinical signs and gross and histologic lesions similar to GE in 6 horses on a single farm. Natural or experimental infection of horses with Mycobacterium avium subsp paratuberculosis has resulted in microscopic, granulomatous, intestinal lesions similar to those found in Crohn’s disease. Some controversial evidence indicates that Crohn’s disease in humans is caused by M avium subsp paratuberculosis. The DNA of this organism is commonly found in intestinal mucosal biopsy specimens of patients with Crohn’s disease, leading to speculation that for genetically susceptible individuals, the innate response to mycobacterial exposure is inadequate, permitting persistent mycobacterial infection to become established. Chronic infection might then activate the inflammatory response.
Lymphocytic-Plasmacytic Enterocolitis
Lymphocytic-plasmacytic enterocolitis is characterized by infiltrates of lymphocytes and plasma cells in the lamina propria of the large or small intestine. Lymphocytic-plasmacytic enterocolitis is infrequently reported in horses, but the few reports of this disease indicate that it has no predilection for horses of any age or breed or for either sex. Horses with LPE are usually examined because of weight loss, but some may be examined because of diarrhea or recurrent signs of abdominal pain. Most horses with LPE have abnormal absorption of carbohydrates, but anemia and hypoalbuminemia are not consistent in horses with the disease. Because lymphoid and plasma cells can be found in rectal tissue of horses with various intestinal diseases, including sarcoidosis (granulomatous disease), cyathostomosis, and malignant lymphoma, the finding of lymphocytic proctitis on rectal biopsy, particularly if mild, may not justify a diagnosis of LPE.
Populations of lymphocytes and plasma cells typically found in various segments of normal intestine of humans and some other species, such as the dog and cat, have been described, but only rudimentary information concerning populations of immune cells in the intestine of horses is available. Breed, environment, diet, athletic activity, and parasite burden may affect populations of immune cells in the intestine. The diagnosis of LPE in the horse is, therefore, subjective, and the accuracy of the diagnosis may depend on the experience of the pathologist examining tissues.
In most published reports of horses with LPE, some responded briefly to treatment with parenterally administered dexamethasone, and others even underwent resolution of LPE after cessation of therapy. However, most affected horses were euthanatized because of poor condition or lack of response to therapy, usually administration of a corticosteroid.
In other species, such as the dog, LPE is speculated to represent a nonspecific immune response by the intestine to a variety of etiologic agents that cause intestinal damage. In dogs, distinguishing between gastrointestinal lymphosarcoma and LPE by histologic examination alone is difficult, and it may be that some horses in which LPE is diagnosed actually have lymphosarcoma. Lymphocytic-plasmacytic enterocolitis in the horse, like in the dog, may be a prelymphomatous intestinal disease.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree