Chapter 21 INFECTIOUS PROBLEMS IN THE LAST TRIMESTER OF PREGNANCY
VIRAL AGENTS
Equine Herpesvirus-1 (Equine Rhinopneumonitis)
Equine herpesviruses (EHVs) are ubiquitous alpha viruses that cause respiratory tract disease, abortions, neurological disease, neonatal foal death, and chorioretinopathy. EHV-1 and EHV-4 are the two most studied and clinically significant herpesviruses of horses and are closely related but genetically and antigenically distinct.1 EHV-1 is the predominant type responsible for abortion (usually after the fifth month of gestation), although sporadic abortions have been associated with more virulent strains of EHV-4.2,3 The increased abortigenic nature of EHV-1 is related to its tissue tropism for endothelial cells in addition to respiratory epithelial cells, neuronal cells, and lymphoid cells.2 In contrast, most strains of EHV-4 possess tropism only for epithelial cells and neuronal cells (trigeminal ganglion), limiting their ability to produce a viremia or disease in sites other than that of the respiratory tract (e.g., uterus or spinal cord).2 Strains of EHV-1 can vary considerably in their pathogenicity, and therefore in their abortigenic potential. EHV-1 and EHV-4 replicate in the upper respiratory tract epithelium following inhalation of aerosolized, infective nasal secretions or by direct contact (inhalation or ingestion) with infected fomites. Infected fetuses, fetal membranes, and fetal fluids are other sources of EHV-1. Following exposure, EHV-1 can enter into either lytic or latent cycles of infection. Lytic cycles of infection involve virus replication, leading to nasal shedding, abortion, or ophthalmic or neurological sequelae. Latent infection cycles result when viral DNA is translocated to the nucleus, but transcription and translation of the viral genome are blocked with the exception of the latency associated transcript (LAT) of EHV-1 in the trigeminal ganglion4 and CD8+ leukocytes.5 Establishment of latently infected individuals is a significant feature of the epidemiology of herpesvirus infection, establishing a potential reservoir for EHV within a herd. Viremia following initial exposure or reactivation of latently infected individuals allows translocation of the virus to the placental endothelial cells, with vasculitis being most pronounced during months 5–9 of gestation compared with month 3 of gestation.6 With profound endometrial vasculitis, abortion can occur without fetal infection,7 and in a survey of field cases of abortion, six of nine abortions caused by EHV-1 were “atypical” with no fetal infection.8 Experimentally the time from exposure to abortion ranges from 9 to 29 days7; however, information on the frequency of reactivation of latently infected mares following stresses (e.g., transport, herd movements, weaning, corticosteroids) and the interval to abortion is unavailable.
Abortion caused by EHV-1 is typically sporadic and occurs during the last trimester, with little or no premonitory signs. Respiratory signs are usually not a feature, either in the aborting mare or within a band of mares. Fetuses are typically delivered dead with an intact amnion, or occasionally within an intact chorioallantois. Subsequent fertility following abortion is dependent on the mare’s inherent fertility, and abortion caused by EHV in the following year is rare. Neonatal EHV foal disease is the rare result of either fetal infection near term gestation or infection immediately after birth. Foals are either ill at birth, or develop severe respiratory distress within the first few days of life, with high morbidity and mortality rates.9
Histopathologic examination of the aborted fetus and placenta is the most common method for diagnosing abortion caused by herpesvirus. Eosinophilic intranuclear inclusion bodies in airway epithelial and hepatic cells from fetuses, and vasculitis of the placenta are characteristic findings in hematoxylin and eosin–stained specimens. Other confirmatory tests included immunohistochemistry demonstrating the expression of viral antigens in infected epithelial and endothelial cells in fixed specimens, and in situ hybridization techniques demonstrating the presence of viral DNA. Serology can provide surveillance information on a herd; however, unless longitudinal sampling is possible, serology does not provide confirmatory evidence of herpesvirus as the causative agent of abortion. Complement fixation (CF) tests measure IgM antibodies, which begin to rise within several days of infection. A single high CF titer would provide strong supportive evidence of a recent EHV infection; demonstration of rising CF titers in paired samples is considered diagnostic. Virus neutralization (VN) tests measure predominantly IgG antibodies, which have a later and more persistent rise following infection than do IgM antibodies. Neither CF nor VN can differentiate between EHV-1 and EHV-4; only type-specific ELISAs are able to do so. Previous vaccination further confounds the usefulness of serology in diagnosing herpesvirus abortions. Surveys of broodmares and foals have shown that the seroprevalence of EHV-4 is greater than 99% in foals and mares,10 whereas that of EHV-1 in mares varies from 26.2%10 to >50% at the time of foaling.11,12 With the exception of PCR tests on peripheral blood leukocytes, most latently infected animals are negative with current diagnostic tests, making it extremely difficult to diagnose latent infection. Reactivation of latent infections does lead to positive serology.
For four decades, vaccination has been used to control EHV-1 respiratory infection and abortion. Several of the available vaccines are labeled to provide protection against abortion, and vaccination during the fifth, seventh, and ninth month is recommended. Evidence that vaccination protects against abortion is somewhat conflicting, with field studies reporting a decrease in the incidence of abortion,13 while in an experimental challenge study the same vaccine (Pnemobort K) failed to prevent abortion or reduce viremia.14 Management probably plays a more significant role in reducing the incidence of abortion. Recommendations include hygiene, segregation of the farm population by age, isolation of pregnant mares by similar stage of gestation, attempt to reduce stresses to mares in the last half of gestation, and quarantine of new horses onto the farm.
Equine Arteritis Virus
Equine viral arteritis (EVA) is caused by equine arteritis virus (EAV), a single-stranded, enveloped RNA arterivirus in the family Arteriviridae. It can cause influenza-like illness in adult horses, abortion, and severe respiratory disease in young foals. Edema of limbs, scrotum, mammary glands, and the periorbital region are also described; however, the disease is commonly subclinical in healthy adults.15 The most important routes of transmission are through aerosolized infective respiratory tract secretions from acutely infected horses and venereally from infective semen (see Chapter 10), but vertical in utero transmission and horizontal transmission via infective fomites has also been reported.15 Following infection of mares, geldings, and prepubertal colts, transient shedding from the respiratory tract occurred for 7–14 days and was not detectable in bodily fluids by 28 days after challenge.16 Persistent infection and shedding in stallions occurs 30%–60% of the time17,18 and duration of shedding can range from weeks to years.15 Abortions have been reported to range from 3 to 10 months of gestation, and rates vary considerably from <10% to as much as 60% in field situations, suggesting that there is variation in the abortigenic potential of different strains of EAV.15 Diagnosis in cases of acute EAV infection is by a greater than four-fold increase in titer in acute and convalescent samples (21-28–day interval). Most fetuses and placentas do not have gross or microscopic lesions, but interlobular pulmonary edema as well as vascular changes in placenta, brain, liver, and spleen have been reported.19 Virus isolation, RT-PCR, immunohistochemistry, and fetal serology are used to diagnosis abortion caused by EAV. There is only one approved modified live vaccine (Arvac, Fort Dodge Animal Health, Fort Dodge, IA) in the United States and Canada and is safe and effective in stallions and non-pregnant mares. Animals should be kept isolated for 28 days following vaccination.
BACTERIAL AND FUNGAL AGENTS
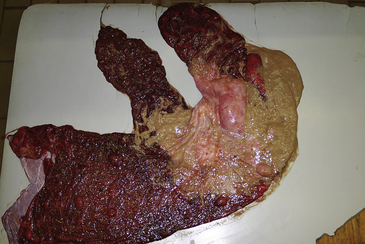
Placentitis
Placentitis has been reported to be responsible for 9.8,20 19.4,21 24.7,22 and 33.5%23 of abortions, stillbirths, and perinatal losses in horses. Fifty-three percent of these losses were due to bacterial infection; Streptococcus equi subsp. zooepidemicus (S. zooepidemicus) was isolated in 28% of these cases.22 Other bacteria frequently identified were Escherichia coli, Leptospira spp. (see below), Crossiella equi (Fig. 21-1), Pseudomonas spp., S. equisimilis, Enterobacter spp., Klebsiella spp., α-hemolytic streptococci, Staphylococcus spp., and Actinobacillus spp.22 The discrepancy in the reported incidences of placentitis lies in a considerably higher occurrence of umbilical cord problems (38.8%) in one of the survey populations,20 compared with 4.5%23 and 3.4%22 in the other populations. The localization of placentitis to the cervical star was present in 95% of cases, supporting the argument that ascension of aerobic bacteria through the vagina and cervix is the most frequent route of infection.24 Clinically, mares may have a vaginal discharge, show udder development, prenatally lactate, and deliver a premature or dead foal. The notable exception to the pattern of ascending infection through the cervix is nocardioform placentitis caused by actinomycetes. The majority of isolates have been C. equi,25 although other species have been identified, including Amycolatopsis spp., Streptomyces spp., and Cellulosimicrobium cellulans. Most reported cases have been from Kentucky, but nocardioform placentitis has also been reported in South Africa, Florida, and Italy.26–28 Inflammation of the chorion extends out from the cranial ventral uterine body, usually at the base of the uterine horn, with an adherent tan to brown mucoid exudate.29 Mares occasionally have precocious udder development, but vaginal discharge is usually not observed. The majority of abortions occur during the ninth and tenth months of gestation. Interestingly, other non-nocardioform bacteria have been isolated from lesions that are grossly indistinguishable from nocardioform placentitis,30 so perhaps the gross appearance of this form of placentitis is unique to its distribution pattern rather than to the causative agent. Nocardioform bacteria have been postulated to be introduced at insemination, then, either because of dormancy or an extremely slow-growing nature, fail to induce a placentitis until late gestation. Attempts to reproduce the disease by inoculation at breeding were not successful (Williams NM, personal communication, 2007).
Sporadic reports of fungi producing abortion include Aspergillus fumigatus and Mucor spp.,31 Allescheria boydii,32 Histoplasma capsulatum,33 Cryptococcus neoformans,34 and Candida albicans.21 Aspergillus spp. are the most common isolates.21,31 The incidence of fungal placentitis is considerably lower than that of bacterial placentitis, although grossly the lesions of the chorioallantois are indistinguishable from those produced by bacterial species. Inflammation of the chorioallantois near the cervical star and fetal emaciation as a result of in utero growth retardation are common features. Granulomatous pulmonary infiltrates are fairly common, but dermatitis is rare.
A model of the pathophysiology of ascending placentitis caused by S. zooepidemicus has been described,35 wherein transcervically inoculated bacteria cross the chorioallantois, colonize the allantoic cavity, gain access to the amniotic cavity via the umbilical cord, and establish a fetal infection by either inhalation or swallowing of bacteria-laden amniotic fluid (Fig. 21-2). The resulting infection and inflammatory cascade is thought to produce observed increases in duration and intensity of uterine contractions,36 elevated allantoic PF2α and PGE2,35 increased expression of the proinflammatory cytokines interleukin-6 and interleukin-8,37 and altered progestin profiles, 38 leading to pre-term delivery of the fetal foal. Whereas the majority (14 of 16) of fetuses were delivered dead or non-viable, the remaining two foals were delivered premature (day 311 and 313), yet viable. These results suggest that acute infection is more likely to result in non-viable offspring, whereas infection of a more chronic nature may be more likely to result in precocious development in utero. Activation of the fetal hypothalamic-pituitary-adrenal axis (HPA), as determined by elevated amniotic cortisol and androgens, has been observed in human infants with infection-induced preterm delivery.39 Studies examining the effect of maternally or intrafetally administered ACTH demonstrated that the equine fetal adrenal gland is relatively insensitive to precocious maturation until very late in gestation.40–42
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree