Chapter 6 Infectious Diseases of the Gastrointestinal Tract
CALVES
Escherichia coli
Septicemia (Septicemic Colibacillosis, Colisepticemia)
Clinical Signs.
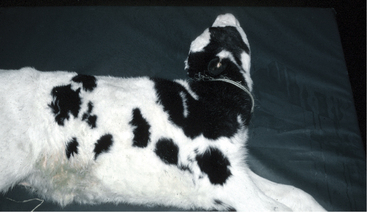
Peracute signs of depression, weakness, tachycardia, and dehydration predominate when highly virulent strains of E. coli cause septicemia. Affected calves usually are less than 7 days of age and may be less than 24 hours old. Although often present early on, fever is usually absent by the time obvious clinical signs of disease occur, when endotoxemia and the resultant poor peripheral perfusion often render the animal normothermic or hypothermic. Exceptions to this rule are calves with peracute disease that collapse when exposed to direct sunlight on hot days—such calves can be markedly hyperthermic. Signs of dehydration are mild to moderate in most cases. The suckle reflex is greatly reduced or absent, and the vasculature of the sclerae is markedly injected. Petechial hemorrhages may be visible on mucous membranes and extremities, particularly the pinnae of the ears (Figure 6-1). The limbs, mouth, and ears are cool to the touch. Affected calves show progressive weakness and lethargy, often becoming comatose before death. Diarrhea is often seen but may not be apparent in peracute cases.
Figure 6-1 Ear of 5-day-old Jersey calf demonstrating petechial hemorrhage associated with E. coli septicemia.
Evidence of localization of infection in certain tissues may become apparent in cases that survive the acute disease. Hypopyon may be present, as may uveitis, which is evidenced by miotic pupils with increased opacity to the aqueous fluid (“aqueous flare”). Hyperesthesia, paddling, and opisthotonus (Figure 6-2) are signs suggestive of septic meningitis. Lameness may result from bacterial seeding of joints and/or growth plates. Signs of omphalophlebitis may be present. Weakness, poor body condition, and recumbency secondary to weakness or joint or bone pain may be present in chronic cases.
Ancillary Data.
Calves suffering from peracute E. coli septicemia often have elevated packed cell volumes resulting from dehydration and endotoxic shock. The total white blood cell (WBC) count is variable but is frequently low or within normal ranges. Generally a left shift is observed, and toxic changes (e.g., azurophilic cytoplasm, nuclear hypersegmentation, and Dohle bodies) are often apparent on cytologic examination of blood neutrophils. Plasma fibrinogen concentration is variable. Hypoglycemia is a common finding, and metabolic acidosis, although common, usually is less severe than in calves recumbent as a result of ETEC. In fact, an acid-base and electrolyte determination that does not demonstrate a severe metabolic acidosis in a recumbent, diarrheic, dehydrated calf less than 14 days of age is a grave sign and usually portends septicemia. Severe hypoglycemia would be the only other laboratory finding that might indicate an easily treatable condition. Blood cultures provide the greatest specific diagnostic aid, but results may not be forthcoming in time to help the patient.
Acute, subacute, and chronic septicemic calves may have detectable clinical signs of localization of infection that allow a more definitive diagnostic test (e.g., cerebrospinal fluid [CSF] tap for patients showing signs of meningitis or joint tap to confirm septic arthritis) (Figure 6-3). In chronic cases, the serum immunoglobulin concentration (and serum total globulin concentration) may be normal or increased as a result of de novo synthesis of antibodies in response to the well-established bacterial infection.
Treatment.
If treatment is attempted, correction of endotoxic shock and acid-base and electrolyte abnormalities, effective antimicrobial therapy, and nutritional support are the primary goals. Intravenous (IV) balanced electrolyte solutions should contain dextrose (2.5% to 10%) and sodium bicarbonate (20 to 50 mEq/L if the plasma bicarbonate concentration is, 10 mEq/L) to address hypoglycemia and metabolic acidosis. Adjustments of the concentration of dextrose and sodium bicarbonate in polyionic fluids can be guided by subsequent serum chemistry results. Maintaining normoglycemia in some peracute and acute septicemic calves can be extremely challenging due to consumption of administered glucose by bacteria. Antimicrobials used to treat neonatal septicemia should be bactericidal and possess a good gram-negative spectrum, such as ceftiofur, trimethoprim-sulfa, or ampicillin. Parenteral administration is necessary to achieve effective blood concentrations. Aminoglycosides such as gentamicin or amikacin can be used alone or in conjunction with the synergistically acting beta-lactam antibiotics (e.g., ceftiofur, penicillin, or ampicillin). The use of potentially nephrotoxic aminoglycosides in a dehydrated patient with prerenal azotemia must be weighed against the potential bactericidal activity of the drugs. Given the present concerns regarding aminoglycoside use in food animals, use should be limited to situations in which other antibiotics have proven ineffective. Further, a minimum 18-month slaughter withdrawal must be enforced for calves that receive aminoglycosides. Use of fluoroquinolones (e.g., enrofloxacin, danofloxacin) in dairy calves is currently not permitted under federal law in the United States.
Prevention: Colostrum and Management.
Enterotoxigenic Escherichia coli
Etiology
Because enterotoxins are nonimmunogenic, efforts to control ETEC in calves have centered on inducing antibody against fimbrial proteins. The type I fimbriae that allow pathogenic ETEC to attach to enterocytes are proteins that initially were categorized with capsular (K) antigens. Currently fimbriae are classified as F antigens. Unfortunately even the current literature still refers to K-99, K-88, and so forth, rather than the current designation, F-5 and F-4, respectively. In calves, F-5 (K-99) is the most commonly identified antigenic type and has received the most attention regarding diagnostics and vaccines for calves. However, ETEC possessing other fimbrial antigens or multiple fimbrial antigens including F-41, F-6, and some types still not widely identified are capable of causing diarrhea in calves. Some ETEC possess more than one type of fimbriae, and both F-41 and F-5 types may be isolated from an ill calf. Colostrum possessing passive antibodies against a specific ETEC fimbriae type will protect the newborn calf against that specific F type but will not cross-protect it against others (Table 6-1).
| Designations | ||
|---|---|---|
| New | Old | Toxin |
| F 4 | K 88 | LT I |
| F 5 | K 99 | STa |
| F 6 | 987 P | STa |
| F 41 | SIa | |
Clinical Signs
Because of the multitude of ETEC types and variability in their pathogenicity, as well as the influence of passive transfer, individual farms may have sporadic or endemic problems resulting from ETEC. Mild disease is common on many farms and seldom is brought to a veterinarian’s attention. These calves have loose or watery feces but continue to nurse (Figure 6-4). Spontaneous recovery or apparent response to the farmer’s favorite “calf-scour” treatment (usually an oral antibiotic) is the rule. Owners usually call for veterinary assistance only when peracute cases develop, a high morbidity is apparent, calves fail to respond to over-the-counter medications, or mortality in neonatal calves is experienced.
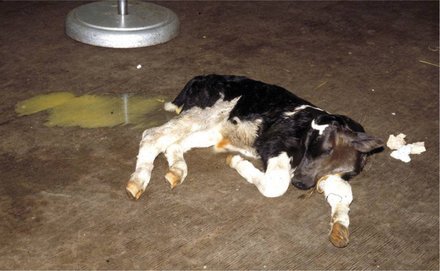
Peracute cases may produce dehydrated, weak, and comatose calves within hours of the onset of the disease. Historically these calves usually have nursed normally and appeared healthy until signs develop. Dehydration and weakness are the predominant signs (Figure 6-5). Mucous membranes are dry, cool, and sticky. The suckle reflex is weak or absent.
Most peracute cases show evidence of voluminous diarrhea (Figure 6-6), with watery feces coating the tail, perineum, and hind legs. Some calves with peracute disease may not have diarrhea; however, the pooling of fluid in the intestinal lumen creates abdominal distention, and fluid splashing sounds can be detected by simultaneous auscultation and ballottement of the right lower abdominal quadrant. Mild, transient colic may be noted early in the disease course. Bradycardia and cardiac arrhythmia accompany the systemic signs in some peracute cases and result from hypoglycemia or hyperkalemia. Atrial standstill has been documented in some bradycardiac calves with hyperkalemia. Rectal temperatures usually are normal or subnormal if the calf is recumbent.
Clinical Pathology
Hypoglycemia is more likely to be present if the interval between feedings is prolonged; this finding is not present in all peracute cases. Blood values for a typical case are shown in Table 6-2. Mild azotemia resulting from prerenal causes (reduced renal perfusion) is common and should be kept in mind when use of potentially nephrotoxic drugs is considered in these patients.
TABLE 6-2 Laboratory Data From a Typical Peracute ETEC Infection in a 7-Day-Old Holstein Calf
| Item Tested | Electrolyte (mEq/L) | Normal Range |
|---|---|---|
| Na = | 127 | 132-150 |
| K+ = | 8.1 | 3.9-5.5 |
| Cl− = | 104 | 97-106 |
| HCO3− = | 12 | 20-30 |
| Tot CO2 = | 10 | 26-38 |
| Ven pH = | 7.09 | 7.35-7.50 |
Treatment
Concentrations of required electrolytes based on subjective clinical parameters rather than objective laboratory tests are empiric at best, but sometimes are necessary in field situations. Therefore rules of thumb include:
Total CO2 of venous blood also may be used to calculate base deficits in lieu of HCO3 values.
Feces usually remain more watery than normal for 2 to 4 days. If diarrhea persists beyond this time, concurrent infection with other organisms is likely. Other treatments for peracute cases may include flunixin meglumine (1.1 mg/kg IV or SQ every 24 hours) directed against potential endotoxemia, resolution of fever, and reduction of pain associated with fluid-filled bowel. Repeated dosages of this product carry the risk of renal and gastrointestinal injury because continued use of flunixin meglumine interferes with vasodilatory prostaglandin synthesis in the gut and kidney.
Prevention
This assumes prime importance when a high morbidity, significant mortality, or both occur on a dairy farm. It is not unusual to encounter 70% to 100% morbidity and mortality when virulent strains of ETEC are present. These strains also tend to be resistant to many antibiotics. The usual situation is that the owner tries multiple over-the-counter products on the first few affected calves and then calls for veterinary assistance to select a “better” antibiotic. One or more calves may die or require intensive therapy before a thorough investigation of the problem ensues.
Other Escherichia coli Diarrhea
Etiology
Shiga-like toxin-producing E. coli (SLTEC) are another type of E. coli that produce hemorrhagic colitis and the hemolytic uremic syndrome in humans. These organisms also have been called EHEC and occasionally have been found in calves. Some of these strains invade the mucosa to reside in the lamina propria of the large intestine and produce a severe hemorrhagic colitis. Ulcerative colitis with hemorrhage may be present grossly and microscopically in necropsy specimens. Those producing Shiga-like toxin (verotoxin) create enterotoxemia, inhibition of protein synthesis, and vascular damage in the involved intestine. Other less common strains of E. coli produce cytotoxic necrotizing factor (CNF), a potent cytotoxin that may be linked genetically to a plasmid that encodes fimbriae and toxins. This plasmid may be found in the same strains of E. coli responsible for calf diarrhea and septicemia in neonatal farm animals.
Prevention
The veterinarian responsible for herd health must consider the public health concerns associated with some AEEC or SLTEC. Currently the 0157:H7 strain has caused a great deal of bad publicity for cattle because cattle have been blamed as carriers of this organism that may infect people, causing severe colitis and occasionally hemolytic uremic syndrome. Therefore sanitation, disinfection, and careful handling of feces to avoid human exposure are indicated.
Rotavirus
Clinical Signs
Signs of depression, dehydration, and shock are more likely to occur in the youngest calves (,5 days of age) and seldom occur in calves more than 2 weeks of age. Recumbent calves usually have profuse watery diarrhea and abdominal distention of the right lower quadrant with fluid-filled small intestine.
Treatment
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree