CHAPTER 33 Infectious Diseases
Fungal and Rickettsial Diseases
Fungal Diseases
Systemic fungal infections are rarely documented in cats. Approximately 7 per 10,000 of the total population of animals presenting to veterinary teaching hospitals in North America are diagnosed with a systemic mycosis.10 In most cases, dogs are the more susceptible species; the exception is cryptococcosis, which is 5 to 6 times more likely to be diagnosed in cats.42 Cryptococcosis, histoplasmosis, coccidioidomycosis, blastomycosis, and sporotrichosis will be discussed separately, and recommendations for treatment will be described at the end of the section.
Cryptococcosis
Of the organisms causing systemic mycosis in cats, Cryptococcus is most commonly diagnosed. In the largest retrospective study evaluating deep mycotic infections in 571 cats, 46.1% of the infections were due to Cryptococcus.10 The organism is round to ovoid in shape, thin walled with diameter of 2.5 to 8 µm (Figure 33-1).17,20 In tissues, it is surrounded by a heteropolysaccharide capsule that varies in thickness depending on the strain and environment. The capsule provides resistance to desiccation and virulence.20 Cryptococcus multiplies asexually with narrow-based budding. The infectious particle, the basidiospore, is adapted to be dispersed by air and has properties that allow it to adhere to and penetrate respiratory epithelium and cause infection.20
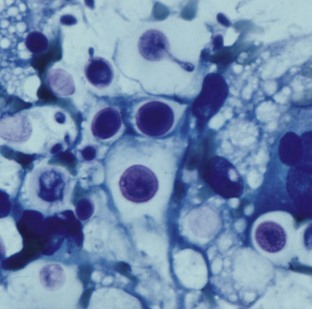
FIGURE 33-1 Cytologic diagnosis of Cryptococcus showing encapsulated yeast forms in a Diff Quik-stained smear.
(Courtesy Richard Malik. [Figure 61-2, B in Greene CE, editor: Infectious diseases of the dog and cat, ed 3, St Louis, 2006, Elsevier.])
Typically, cryptococcosis in people and domestic animals is caused by one of two species: Cryptococcus neoformans (var. neoformans or var. grubii) or C. gattii.20 Globally, C. neoformans var. grubii is the most common isolate associated with disease in people and animals. C. neoformans causes almost all cases in the United States and Europe.20,31 Within the United States, the highest incidence of cryptococcosis in cats is reported in California, Florida, Virginia, and Iowa.10 C. gattii most commonly causes disease in tropical and subtropical areas, including Australia, Papua New Guinea, Southeast Asia, and Central Africa; it has also been documented to cause infection in the temperate climate of the Pacific northwest, including Vancouver, Canada.37 C. albidus was confirmed to cause one case of systemic disease in a cat in Japan but has not been recognized as a significant pathogen in cats.31
Cryptococcus can be isolated from a variety of substances, depending on the geographic location of the organism as well as the species.42 C. neoformans is consistently found in pigeon feces and soil enriched by avian feces and less often in milk, fermenting fruit juices, air, dust, wasp nests, grass, and insects.20,42 C. gattii is found in hollows of Eucalyptus and fig trees in Australia and some fir trees in western Canada.20,42 Risk factors for C. gattii infection in pets in Vancouver include proximity to logging sites or other areas of commercial soil disruption and owners hiking or visiting a botanical garden.14 It is viable in feces for up to 2 years in moist environments.20 Ultraviolet light and dry conditions can decrease viability.
The exact mode of transmission is unknown, but most likely occurs by inhalation of yeast cells or basidospores.13,20 Once inhaled, Cryptococcus lodges in the nasal passages and causes mycotic rhinitis; lower respiratory infection is uncommon because most organisms are larger than the alveolar diameter of 2 µm.17 Some strains are particularly virulent and will destroy adjacent facial bones and spread locally.20 Rarely, cryptococcosis occurs secondary to a penetrating skin wound, causing localized infection.37
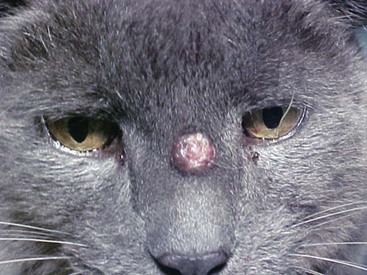
Clinical signs depend upon location of infection. Infection usually involves the nasal cavity, skin, subcutis, central nervous system (CNS), and regional lymph nodes. Dissemination has been documented (Figure 33-2). As mycotic rhinitis of the rostral nasal cavity occurs most often, sneezing, wheezing, and unilateral or bilateral nasal discharge are common presenting complaints. Respiratory signs have been reported in 26% to 83% of cats with cryptococcosis.12,39 In another study, 63% of 263 cats had nasal discharge, and 12.5% had cough or dyspnea.10 Thickening and inflammation of nasal mucosa or nasal granulomas may be visible. Osteomyelitis may occur, leading to facial deformity, including broadening of the nose or swelling of adjacent tissue (Figure 33-3). If the nasopharynx is affected, clinical signs may be absent until the infection spreads through cribriform plate and causes meningitis.38 Alternatively, cats may present dyspneic or with stertorous breathing because of obstruction by fungal granulomas. Lymph node involvement was present in 39% of 263 cats.10 Mycotic pneumonia and hilar lymphadenomegaly because of Cryptococcus is rare in cats.
Cutaneous nodules were documented in 41% of 263 cats with cryptococcosis (Figures 33-4 and 33-5).10 Oral lesions occasionally occur in cats with Cryptococcus infection and may appear as diffuse ulceration of the oral mucosa of the tongue, gingiva, or palate, or as proliferative lesions.43 Central nervous system involvement also occurs, either because of erosion of nasal infection through the cribriform plate or possibly by hematogenous spread. Neurologic signs occur in 8% to 26% of cats with cryptococcosis and may manifest as blindness, pupil changes, ataxia, depression, and temperament changes.10,12,41
Cryptococcosis has been diagnosed in cats less than 1 month of age and in those greater than 15 years of age. The mean age at diagnosis is about 6 years, with 58% of cats being between 2 to 7 years of age.10,17 Some, but not all studies, have shown breed predisposition, with Abyssinian, Siamese, Birman, and Ragdoll cats being overrepresented compared with domestic shorthairs.10,17,42 Indoor as well as outdoor cats are susceptible to infection. A gender predisposition for cryptococcosis in male cats is inconsistently reported. Cryptococcosis occurs most commonly in immunocompromised people, but most studies of the infection in cats do not show an association with retrovirus infection or other causes of immunosuppression.20
A definitive diagnosis of cryptococcosis requires culturing the organism from infected tissue (Box 33-1). Both C. neoformans and C. gattii can be cultured from the nasal cavity of asymptomatic patients. Culture of most systemic fungal infections is laborious and poses a zoonotic hazard to laboratory staff. Culture of Cryptococcus on Abouraud dextrose agar may take up to 6 weeks to be evident.17 In most cases, a presumptive diagnosis is made by cytologic evaluation. The organism may be detected from nasal swab samples, nasal washing, and nasal tissue biopsy imprint or from aspiration of other infected tissues. Rhinoscopy or advanced imaging may aid in diagnosis. If ocular involvement is present without evidence of disease elsewhere, vitreous or subretinal fluid may be aspirated for cytologic evaluation. Quik-Dip (Mercedes Medical, Sarasota, Fla.), Wright Giemsa, or new methylene blue stains enhance visualization of the thickly encapsulated, broad-based budding yeast cells. If cryptococcosis cannot be confirmed cytologically or histopathologically, then serology can be performed. The antigen latex agglutination test is highly specific and sensitive in detecting Cryptococcus capsular antigen in dogs. It can be performed on serum, cerebral spinal fluid, or vitreous fluid. The specificity and sensitivity has not been described in cats, but infected cats can have extremely high titers (>1 : 65,536).37 Serial serologic testing can be used to assess response to treatment, and a favorable prognosis often accompanies a decrease in titer.
Overall, the prognosis for cats with cryptococcosis is good, if the disease is not severe, there is no CNS involvement, and treatment is of appropriate duration. Animals presenting with or progressing to CNS disease are 4 times more likely to die than those without CNS signs.12 In one retrospective study of 59 cats from Sydney, Australia with cryptococcosis, 76% were successfully treated.41 Determining the ideal duration for treatment can be difficult. It is typically recommended to treat for at least 1 month past clinical resolution, and sometimes therapy is needed for 9 months or longer. If fungal granulomas are present in the nasal cavity or nasopharynx, debulking the abnormal tissue may aid in treatment. Itraconazole is the drug most commonly used for treatment of feline cryptococcosis, but other azoles as well as amphotericin B have been used successfully.37
Histoplasmosis
The second most common cause of systemic mycosis in cats is due to Histoplasma; 16.7% of 571 cats diagnosed with systemic fungal disease had histoplasmosis in one study.10 Histoplasma has a global distribution. Pathogenic species include H. capsulatum, H. duboisii, and H. farcinminosum. H. capsulatum causes infection in the continental United States, and H. duboisii is the causative agent in Africa. The organism thrives in warm (22° C to 29° C) and moist environments, particularly in temperate and subtropical areas.22 H. capsulatum is most commonly isolated from moist, nitrogen-rich soil containing bird or bat feces.20,32 In the environment, the organism exists as a mycelial form and within a host as a yeast.
H. capsulatum is most commonly diagnosed in North and South America, India, and southeastern Asia, although it has been documented in every continent with the exception of Antarctica.22 H. capsulatum is endemic in the U.S. Midwest, South and areas along the Ohio, Mississippi, and Missouri rivers. It is sporadically reported elsewhere, including California, Ontario, Canada, and Australia.7 Although reported in 31 states within the United States, the highest incidence is in Oklahoma, Texas, Virginia, and Louisianna.10,22
The life cycle of H. capsulatum is similar to other dimorphic fungi. The mycelial stage living in soil is resistant to environmental damage. It sporulates at temperatures around 22° C, and the spores are known as microconidia or macroconidia.22 Lower respiratory infection most likely occurs because of inhalation of infective microconidia. Some cases of histoplasmosis are isolated to the gastrointestinal (GI) system, but an oral route of infection has not been confirmed.22 At body temperature (37o C), the inhaled organism transforms into the yeast phase within the lungs, is phagocytized, and replicates intracellularly. Infection may be limited to the lower respiratory system and regional lymph nodes, or yeast-laden macrophages may disseminate the organism via lymphatics or hematogenously. In most patients, cell-mediated immunity is effective in controlling the infection; however, if a large number of organisms are inhaled or if immune compromise exists, severe disease may occur.22 In patients with an effective immune system, dormant infections may be reactivated because of immunocompromise.
Unlike other systemic fungal infections, histoplasmosis occurs equally in dogs and cats.32 Histoplasmosis has been diagnosed in cats less than 8 weeks of age to more than 15 years, and it occurs most commonly in cats less than 4 years of age.10,32 The mean age at time of diagnosis is 3.9 years.7 Both outdoor and exclusively indoor cats are at risk of infection.10,30 No consistent gender bias is reported. Breed predilection is not consistently described, but Persian cats were predisposed in one report.10 Although one retrospective study found that concurrent infection with feline leukemia virus (FeLV) was present in 15% of 96 cats with histoplasmosis, in most reports co-infection with feline retroviruses is uncommon.10,29,30,32
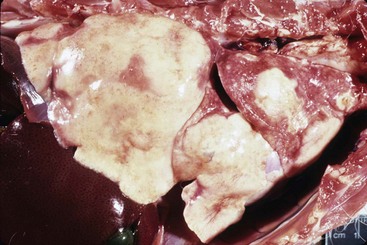
Although infection with H. capsulatum may be asymptomatic and self-limiting, dissemination is common in cats and may occur in up to 95% of cases, despite a lack of systemic clinical signs.10 The organs most commonly affected include the lungs (Figure 33-6), GI tract, lymph nodes, spleen, liver, bone marrow, eyes, and adrenal glands. The incubation period is approximately 12 to 16 days in people and dogs and is likely the same for cats.10,22 Clinical signs are often present for 2 to 3 months prior to presentation.29 In 96 cats with histoplasmosis, the most common clinical signs, which occurred in 67% of infected cats, were nonspecific and included lethargy, weakness, and fever.10 Respiratory signs were present in 39% of the cats and ocular in 24%. When pulmonary involvement is present, clinical signs may include abnormal lung sounds, tachypnea, or dyspnea. Ocular abnormalities included blepharitis, conjunctivitis, anterior uveitis, chorioretinitis, optic neuritis, and retinal detachment.10,22 Lymphadenopathy and hepatosplenomegaly can occur with dissemination. If present, bone marrow involvement may lead to cytopathies.32 Uncommon sites for infection include skin, bone, CNS, and oral cavity.33,53 Specific GI signs occur less commonly in cats than in dogs. Oral infection may manifest as ulcerated tissue or proliferative lesions on the gingiva or palate.33
Routine laboratory tests are often abnormal in cats with histoplasmosis, but findings are not pathognomonic for infection. Anemia, thrombocytopenia, leukopenia, or pancytopenia may be present. The most common hematologic abnormality is normocytic, normochromic, nonregenerative anemia, which may be due to chronic inflammation, bone marrow involvement, and GI blood loss. Neutrophilic leukocytosis with monocytosis can also be diagnosed, or the leukogram may be normal. The organism can be seen in phagocytic cells on blood smears, and in a study of 56 cases, it was evident in 20% of the cats with histoplasmosis.10 Other abnormalities reported include thrombocytopenia and severe pancytopenia.22 Biochemical abnormalities have included hypoalbuminemia, hyperglobulinemia, elevated liver enzyme activity, hyperbilirubinemia, and hypercalcemia.32 In one report, radiographic abnormalities were present in over 87% of cats with histoplasmosis, and the most common finding was a diffuse or nodular interstitial pattern in the lungs.10 Hilar lymphadenomegaly was rare. Abdominal fluid, hepatomegaly, or splenomegaly may be present.
Definitive diagnosis of histoplasmosis requires identification of the organism cytologically or histologically. Histoplasma is usually present in clusters within cells of the mononuclear phagocyte system in the infected organs. When stained with Wright or Giemsa, H. capsulatum appears as a small (2 to 4 µm) round body with a basophilic center and lighter halo caused by shrinkage of the yeast during the staining process.22 Diff-Quik can also be used to stain cytologic preparations. In cats, the organism is most commonly found by fine-needle aspirate cytology of infected organs, including lung, lymph node, dermal lesions, spleen, liver, or bone marrow. Organisms may be seen on evaluation of fluid collected by endotracheal wash, bronchoalveolar lavage, thoracocentesis, or cerebrospinal tap. Histopathologic abnormalities include granulomatous inflammation, but H. capsulatum may be difficult to see with routine staining. If fungal disease is suspected then special stains, such as periodic acid–Schiff (PAS), Gomoris methenamine silver, or Gidley fungal stain, should be requested. Immunostaining has been used to diagnose histoplasmosis in skin biopsy samples.22
Diagnosis of histoplasmosis by culture of the organism is rarely performed because of the zoonotic risk for laboratory personnel; it can also take up to 4 weeks for results to be available. Serologic testing is unreliable and false negatives are common in animals with clinical disease, and false-positive results may occur in previously infected patients with residual antibodies.22 MiraVista Diagnostics (Indianapolis, Ind., www.miravistalabs.com) has developed a test that can detect H. capsulatum antigens in urine, serum, or cerebrospinal fluid (CSF); this has been used to diagnose histoplasmosis in people. Sensitivity is increased if both urine and serum is tested. The test can be used to monitor response to treatment; titers decrease with effective therapy and increase with disease relapse. In people, cross reactivity occurs with blastomycosis, coccidioidomycosis, and penicilliosis. At the author’s institution, the urine antigen test has been used for monitoring purposes in dogs confirmed to have histoplasmosis, based on cytologic diagnosis. The test is likely applicable in cats, but more research needs to be performed to determine sensitivity and specificity.
Although histoplasmosis may be self-limiting when isolated to the lungs, treatment is recommended to avoid dissemination. As with most systemic fungal diseases diagnosed in cats, itraconazole is the medication of choice. Prognosis varies depending on the extent of disease. Of 56 cats in which the outcome was known in one study, 68% died or were euthanized.10
Coccidioidomycosis
Coccidioidomycosis was diagnosed in 9.2% of cats with systemic fungal disease in one study.10 Coccidioides is a dimorphic fungus that grows in soil as a mycelium. Mycelia germinate to form thick-walled, barrel-shaped, rectangular, multinucleate arthroconidia that are 2 to 4 µm wide and 3 to 10 µm in length.24 Mycelium can persist in soil indefinitely and arthroconidia are environmentally resistant. When soil containing Coccidioides is disturbed, arthroconidia are released and dispersed.24 They can germinate and produce new hyphae or serve as a source of infection.
Coccidioides is found in a specific ecologic location, known as the Lower Sonoran life zone.24 This area includes the southwestern United States, Mexico, and Central and South America.21 It is also known as valley fever. These regions have sandy, alkaline soil, and high temperatures, with the summer mean greater than 26.6° C and the winter mean 4° C to 12° C.24 In addition, elevation and annual rainfall are low. During prolonged periods of dry, hot weather, Coccidioides survives below the soil as deep as 20 cm.24 After rainfall, the organism replicates near the soil surface and releases large numbers of infective arthroconidia that disseminate.24
Infection is most common when the soil is dry and is disturbed, such as by dust storms, earthquakes, or crop harvesting, or following the rainy season.21 C. immitis is the species found in California in the San Joaquin Valley, while C. posadasii is found in all other endemic areas.24 Disease is most common in California, Arizona, and southwestern Texas and less commonly diagnosed in New Mexico, Nevada, and Utah. Infections outside of endemic areas are sporadic. In such cases, the individual may have traveled to an endemic area and then had activation of dormant organisms years later.24 In people, most infections are asymptomatic, with only 40% of people developing clinical signs.24 This may be true in other species.
Coccidioidomycosis occurs primarily following inhalation of infective arthrospores, and less than 10 organisms can cause infection.21 Uncommonly, infection will occur following inoculation of the organism into the skin. There is one case report of a veterinary assistant developing coccidioidomycosis after being bitten by an infected cat.16 Rarely, there have been suspect cases of dogs becoming infected after contacting fomites contaminated with arthrospheres.21
Once inhaled and spread to the alveoli, arthroconidia convert to spherules because of the higher temperature and increased carbon dioxide level.21,24 Once the spherule matures, it eventually ruptures, releasing up to 300 endospores.24 After inhalation, it takes approximately 3 days for the endospores to form, but clinical signs usually do not occur for 2 weeks.21 It is thought that endospores are able to disseminate through blood and lymphatics to distant sites, and cats with disseminated disease may have no infection in the respiratory system.49 When skin inoculation occurs, infection may be limited to the dermis or subcutis.
As is typical of fungal disease, cell-mediated immunity is much more effective in resolving infection with Coccidioides than is humoral immunity. Antibody formation typically occurs but is more useful as a diagnostic aid than in fighting the infection. Although people who recover from coccidioidomycosis are considered immune to reinfection, recurrence in dogs and cats is common. It is not known if this is due to premature discontinuation of treatment or to a lack of long-term immunity to Coccidioides.21
Clinical disease varies from subclinical to fatal; it is not known why some infected animals have self-limiting disease and others die despite treatment. Evaluation of necropsy reports from 1995 to 2005 from the Arizona Veterinary Diagnostic Laboratory found that in fatal coccidioidomycosis cases, 25% of the animals involved were cats. It is unclear if this finding is due to more severe disease in cats or if coccidioidomycosis is underdiagnosed antemortem in cats. Infection is most commonly reported in middle-aged cats, with no breed predisposition. There is also no correlation between coccidioidomycosis and feline retrovirus infection.21
Specific clinical manifestations depend on the site of infection and are extremely varied, making early diagnosis a challenge. Coccidioides appears to be able to infect most tissues. Fever is commonly present at diagnosis, as well as nonspecific signs, including lethargy, anorexia, and weight loss. Respiratory signs are uncommonly recognized, and lung and bone involvement occurs less frequently in cats than in dogs. Ocular lesions, including chorioretinitis and anterior uveitis, occur with similar frequency among cats and dogs.24 In cats, approximately 50% of cases are disseminated.21 Subclinical infection may occur in up to 70% of dogs; it is unknown if this is true in cats.21
Coughing is uncommon, but 25% of cats may present dyspneic. The CNS can be infected, and granulomatous mass lesions in the brain are more common than fungal meningitis. Ocular signs may include anterior uveitis, subretinal granulomas, retinal detachment, and blindness. There is also a report of cats that presented for periocular swellings with systemic signs, including weight loss, unkempt hair coat, and lethargy. Clinical ophthalmologic abnormalities were bilateral in each cat and included hyperemia, conjunctival masses, fluid-filled periorbital swellings, granulomatous chorioretinitis, nonhematogenous retinal detachments, and anterior uveitis.51 Cats were diagnosed with coccidioidomycosis using a combination of clinical findings, serology, and, in two cases, visualization of Coccidioides spherules by either aspiration cytology or biopsy. Active anterior uveitis and periocular swelling resolved with treatment. Chorioretinal granulomas, although persistent, significantly decreased in size.51
Infection is often limited to the lungs and perihilar lymph nodes, although dissemination of the organism through the blood and lymphatics can occur.21 When dissemination occurs, the skin is the most frequent site of infection. In 15 cats with coccidioidomycosis that underwent post-mortem evaluation, all had multiorgan involvement.21 Nonhealing cutaneous lesions, including abscesses, dermatitis, chronic draining tracts, and ulcerations are the most common clinical manifestations.21 Cutaneous and subcutaneous lesions were reported in 56% of cats in one series of 48 cases.49
Cytologic confirmation may be made by evaluation of aspirates of affected lymph nodes, skin lesions, or lungs. The organism can be seen on unstained slides and appears as a large (10 to 80 µm) round, double-walled structure containing endospores.24 Multiple biopsy samples may need to be collected and evaluated in order to see the organism histologically. Although spherules can be detected with routine hematoxylin and eosin stain (H&E), they are easier to see when PAS or Grocott-Gomoris methenamine silver stain is used. Commercial laboratories that practice biosafety precautions can isolate Coccidioides on culture. The mycelia that grow on culture media are highly infectious. Serologic testing using tube precipitin and complement fixation techniques were useful in 48 cats with coccidioidomycosis.24
Itraconazole is typically used for treatment of feline coccidioidomycosis. Of 53 cats diagnosed with coccidioidomycosis, 67% survived with treatment.10
Blastomycosis
Blastomyces dermatitidis is the saprophytic dimorphic fungus that causes blastomycosis. It exists in a mycelial form and reproduces sexually, producing infective spores. At body temperature, the spores transform into yeast and replicate asexually. Budding yeasts are 5 to 20 µm in diameter and have a thick, refractile, double-contoured cell wall.36 Dogs and people are the species infected most commonly, but blastomycosis has been reported in other animals, including bats, horses, sea lions, wolves, ferrets, and nondomestic cats.19,36 It is rarely reported in domestic cats.
The most likely reservoir for Blastomyces is soil. Because normal soil organisms destroy most Blastomyces organisms, specific environmental conditions are needed for Blastomyces to survive. Blastomyces thrives in a sandy, acidic soil near water. It has also been isolated from decaying wood and vegetation, animal excrement, a beaver dam, and animal waste.19,36 Even in endemic areas, blastomycosis occurs in geographically restricted foci. Living near water is a risk factor for blastomycosis in dogs. In people, disturbing soil is associated with infection, and precipitation may facilitate the release of infective spores. Most people and dogs are likely exposed to Blastomyces on their own property, because there has been documentation of repeated cases occurring at the same location despite occupation by multiple families.3 The source of infection in cats is unclear. Many reported cases of feline blastomycosis have occurred in strictly indoor cats. One study analyzed 60 environmental samples obtained from four homes in which blastomycosis was diagnosed in cats, and all were negative for B. dermatitidis.3,19
During a 10-year period, eight cases of blastomycosis were diagnosed in cats at the veterinary teaching hospital (VTH) in Illinois, while during a span of 11 years, 5 cats were diagnosed with blastomycosis at the VTH in Tennessee.3,19 The prevalence of canine blastomycosis in Tennessee between 1979 and 1989 was 1.2%, compared with less than 0.1% in cats.4 Of 571 cats diagnosed with systemic mycosis, 41 (7.1%) were infected with B. dermatitidis.10 B dermatitidis mainly causes disease in the United States and Canada, but is endemic in Africa and India and has been documented in Europe and South America.6 Within North America, endemic areas include the Mississippi, Missouri, and Ohio river valleys; the mid-Atlantic states; and the Canadian provinces of Manitoba, Ontario, and Quebec.36 Blastomycosis has also been diagnosed in New York, Wyoming, South Dakota, Colorado, and Saskatchewan.6,27,36 In a retrospective review of 41 cats diagnosed with blastomycosis, most cases occurred in Oklahoma, Tennessee, and Wisconsin.10 Risk factors and the epidemiology of blastomycosis are unknown in cats because of its rarity.
There are so few cases described in the literature that it is not possible to determine predisposing factors. Many reports provide conflicting data. Most cases described in the literature were diagnosed at necropsy.19 Males may be slightly predisposed; one study reported that 69% of 36 infected cats were male.3 Breeds described as being predisposed include Siamese, Abyssinian, and the Havana Brown, but this is not supported in all reports.10,19 Affected cats have ranged in age from 6 months to 18 years.4 The typical age of infected cats varies among publications: In three studies, 75% were less than 4 years of age, 42% were less than 4 years, and 87% of cats were greater than 7 years.19 Duration of clinical signs prior to diagnosis has ranged from 3 days to 7 months.10,19 A history of immunosuppression is rarely present; 10% of 41 cats with blastomycosis were FeLV positive, none were FIV positive, and one was positive for feline infectious peritonitis (FIP).10
Based on thoracic radiographs and necropsy data, infection in cats occurs most commonly in the lung, even though respiratory signs may be absent (Figure 33-7).19 B. dermatitidis has been documented in lymph node, kidney, eye, CNS, skin, GI tract, pleura, peritoneum, heart, liver spleen, trachea, and adrenal glands.19,36 Infection is frequently disseminated. Clinical signs vary depending on the site of infection. The most commonly reported clinical signs in cats with blastomycosis vary among publications, but dyspnea, lethargy, weight loss, and fever are frequently present.19 Other reported respiratory signs have included cough, tachypnea, sneezing, and increased bronchovesicular signs.19 Central nervous system, ocular, and dermatologic involvement may manifest clinically as well. Ocular changes described include retinal granulomas and detachment, chemosis, corneal edema, and uveititis.6,7,19 Skin lesions may be draining tracts or nonulcerated dermal masses and range from a few millimeters to a few centimeters in diameter.19
Diagnosis of blastomycosis can be difficult in cats, particularly because there are no pathognomonic clinical manifestations. Hematologic and biochemical changes are neither specific nor consistent among infected cats but may include anemia, leukopenia or leukocytosis, monocytosis, hyperglobulinemia, hypoalbuminemia, and hypercalcemia.19 Radiographic changes may include poorly defined soft tissue opacities with nodules or masses or alveolar lung consolidation and pleural effusion.10,19
Definitive diagnosis is made by cytologic or histologic identification of B. dermatitidis. Pyogranulomatous inflammation is commonly seen associated with large numbers of broad-based budding yeasts. Diagnosis has occurred by cytologic exam of fine-needle aspirate samples of infected skin, draining tracts, lymph nodes, and lung. Bronchoalveolar lavage can also be performed to diagnose pulmonary blastomycosis.19 In dogs, the sensitivity and specificity of the agar-gel immunodiffusion test (AGID) was reported as 91% and 96%, respectively. The usefulness of AGID testing for blastomycosis in cats is unknown. Of three cats with blastomycosis tested using AGID, one was positive.10 MiraVista Diagnostics offers an antigen test for B. dermatitidis that has been validated in dogs and has the greatest sensitivity when urine is tested.50 It is unknown if this test is sensitive or specific in cats.
Currently, treatment with itraconazole is recommended.6 The prognosis is guarded to poor. Of four cats treated for blastomycosis, three died within 12 days of diagnosis.3
Sporotrichosis
Sporotrichosis is a mycotic disease of humans and many animal species caused by the dimorphic fungus Sporothrix schenckii, which is endemic worldwide. Zoonotic transmission of S. schenckii between cats and people has been documented and is considered an emerging zoonosis.11,45,48,54,55 S. schenckii survives in the environment, typically in decaying vegetation, and people and animals are infected by wound contamination or penetrating foreign bodies. The organism becomes pathogenic because of its dimorphic abilities. After entering the skin through a puncture, bite, or scratch, the fungus converts to a yeast phase. The organism has also been isolated from the nails and oral cavity of cats and presumably can be inoculated into bites, scratches, or puncture wounds.47,48 Three clinical syndromes of sporotrichosis are known in cats:
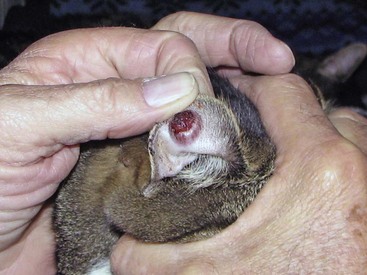
The localized and lymphocutaneous forms are the most common. Cutaneous lesions are most commonly found on the face, nasal planum (Figure 33-8), tail base, and legs and may be solitary or multiple. Lesions appear after an incubation period of about 1 month and first appear as draining puncture wounds mimicking bacterial fight-wound abscesses or cellulitis. Treatment with antibiotics does not result in resolution. The lesions may then become ulcerated and form large, crusted areas. The localized form may progress to the lymphocutaneous form, especially if not treated. In the lymphocutaneous form, cutaneous nodules may progress to draining ulcers of the skin, subcutis, and lymph nodes. The disseminated form is primarily found in the liver and lungs, but involvement of other organs has been documented.
Outbreaks of sporotrichosis are thought to be rare. In a large series of 347 cats with naturally acquired sporotrichosis in an epidemic in Rio de Janeiro, the median age was 2 years, and cats of male gender predominated.46 Most cats were infected through fight wounds, and multiple skin lesions were common. Most lesions were on the head. The skin lesions were varied and included small crusted lesions, subcutaneous nodules that progressed to draining lesions and ulcers, extensive exudative ulcers, and extensive zones of necrosis that exposed muscle and bone. More than 25% of cats had lymphangitis and regional lymphadenitis. The most common extracutaneous signs were respiratory signs, such as sneezing and dyspnea. Subclinical infections were also documented.
It does not appear that infection with FeLV or feline immunodeficiency virus (FIV) is a predisposing factor for sporotrichosis in cats.46,54 Concurrent infection with FIV does not affect clinical outcome.46
Diagnosis of sporotrichosis in cats is most often by cytologic examination of exudates and aspirates from abscesses or nodules or impression smears from skin lesions. Smears stained with a Romanowsky-type stain typically contain large numbers of yeastlike organisms that are often cigar shaped but may appear as round budding shapes. Histopathology is not a reliable method of diagnosis; in two published case series, the organism was not present in more than 1 of 3 affected cats.9,46 Failure to find the organism in biopsy specimens may be due to sampling early in infection or individual variation in immune response. Definitive diagnosis is by fungal culture of exudate from deep within a draining tract and/or macerated tissue samples.
The drug of choice for sporotrichosis in cats is oral itraconazole. Ketoconazole and sodium iodide have also been reported as effective treatments, but the rate of adverse effects is high.46,54 Successful treatment of localized disease with a combination of oral itraconazole and intralesional amphotericin B has been described.25 Secondary bacterial infections should be treated according to culture and sensitivity results for 4 to 8 weeks. Antifungal treatment should be continued for 1 month past resolution of clinical signs to prevent recurrence. Treatment may be required for months to over 1 year; therefore client compliance may be an obstacle to achieving cure even though the prognosis is good. People handling cats suspected or confirmed with sporotrichosis should wear gloves as well as wash their hands and arms with a disinfectant scrub.
Antifungal Therapy
The best therapy for management of systemic fungal disease in cats is ultimately dependent on the individual patient. Preexisting medical conditions, site of fungal infection, and cost of therapy are factors to consider when choosing treatment (Table 33-1).
TABLE 33-1 Drugs for Treatment of Systemic Fungal Infections in Cats
| Drug | Dose | Comments |
|---|---|---|
| Amphotericin B | Original formulation: 0.5 mg/kg, IV, 3 times/week Lipid mixture (Abelcet): 1 mg/kg, IV for 2 hours, 3 times/week | Cumulative dose in cats should not exceed 4 to 6 mg/kg for the original formulation, and 12 mg/kg for the lipid mixture |
| Flucytosine (Ancobon) | 50 mg/kg, PO, q8h | Used in combination with amphotericin B |
| Itraconazole (Sporanox) | 10 mg/kg, PO, q24h | Administer capsules with food; administer liquid on empty stomach |
| Fluconazole (Diflucan) | 30-50 mg/cat PO q12h 75 mg/cat PO q12-24h |
Note: Duration of treatment is difficult to determine but should be at least 1 month past clinical resolution.
Amphotericin B is fungicidal and causes cell death by binding to ergosterol in the fungal cell membrane and disrupting membrane stability. It has a broad spectrum of efficacy against many fungal species and was initially the treatment of choice for systemic mycosis in people and animals. It has been proven to eliminate fungal meningitis.37 Its potential for nephrotoxicity limits the total dose that may safely be administered to a patient, and its use in patients with compromised renal function is not recommended. Newer formulations are safer but more expensive. The three types of newer formulations of amphotericin B include a lipid mixture (Abelcet), a colloidal suspension (Amphotec), and a liposome-encapsulated form (AmBisome). The lipid complex is the least expensive and has been used the most in veterinary medicine.26 It is 8 to 10 times less nephrotoxic than the original amphotericin B, when administered to healthy dogs.26 The new formulations are taken up rapidly by the reticuloendothelial system, leading to the high drug levels in infected organs, including the liver, spleen, and lungs.26 A higher cumulative dose of the new formulations may be administered without increasing risk of drug uptake by the kidneys and nephrotoxicity. The lipid-complexed amphotericin B has been used successfully in veterinary patients for treatment of cryptococcal meningitis, histoplasmosis, coccidioidomycosis, blastomycosis, and other systemic mycoses.
Indications for use of lipid complexed amphotericin B include cryptococcosis with CNS involvement, in mycotic infections that are severe or progressive and in cats that cannot tolerate oral administration of antifungal agents. An appropriate dose of Abelcet in cats is 1 mg/kg intravenously spanning 2 hours. Therapy is administered 3 times weekly for an accumulative dose of 12 mg/kg.26 If the original formulation of amphotericin B is used, a dose of 0.5 mg/kg IV 3 times weekly has been recommended.37 Monitoring for changes in renal function, such as creatinine, blood urea nitrogen (BUN), and glucosuria, is indicated. Amphotericin B may also be effective as a fungicide by causing immunomodulation and activating macrophage uptake and killing of fungal organisms.26 Amphotericin B combined with flucytosine may provide the greatest efficacy when treating cats with disseminated disease and/or CNS involvement. This combination is considered by some as the treatment of choice for feline cryptococcosis.37
Flucytosine is rarely used as sole therapy, but is combined with other antifungals to increase efficacy. It is synergistic when combined with amphotericin B and penetrates the blood–brain barrier. It has been associated with drug reactions in dogs, and its use may be limited to the first 10 to 14 days of treatment.37
Azole antifungals inhibit ergosterol biosynthesis, interfering with fungal membrane function.26 One benefit of azole drugs is that they allow for treatment of patients without hospitalization. Itraconazole is considered the drug of choice for treatment of most cases of systemic mycoses that are not immediately life threatening in cats.26,37 It does not easily cross the blood–brain, blood–eye, or blood–prostate barriers. Although it does not penetrate these organs well, it has been used successfully to treat fungal meningitis in cats. Such success may be due to a decrease in the blood–brain barrier associated with inflammation. It is more effective than ketoconazole and has fewer side effects. Side effects can include GI upset, hepatic disease with elevations in alanine aminotransferase activity, and rarely, cutaneous lesions resulting from vasculitis. The capsule formulation of itraconazole should be administered with food to increase absorption, while the liquid formulation should be given after a fast. It should not be administered with antacids. Itraconazole is typically dosed at 10 mg/kg PO q24h for treatment of cryptococcosis, histoplasmosis, coccidioidomycosis, and blastomycosis.6,22,37
Fluconazole is effective in treatment of systemic mycoses, particularly when there is involvement of the CNS, eye, or urinary system. It may be the most effective antifungal for treatment of feline cryptococcosis.37 It has also been used in cats that cannot tolerate itraconazole or in which itraconazole is ineffective. Published doses include 30 to 50 mg/cat PO q12h and 75 mg/cat PO q12-24h.37 Ketoconazole is not considered a drug of choice for management of feline fungal disease but has been used successfully in treatment of C. gattii. It has a higher rate of side effects and is less efficacious than itraconazole.
Newer azoles are on the market and are being used in veterinary medicine. Voriconazole is a derivative of fluconazole but has greater potency and a broader spectrum of activity. It is highly bioavailable when administered orally and also comes in an IV formulation.26 Posaconazole is an itraconazole analog. It has been used successfully in animal models for the treatment of systemic histoplasmosis and coccidioidomycosis, as well as cryptococcal meningitis.26 There is little published data describing use of these newer azoles in cats.
Rickettsial Diseases
Rickettsia are obligate intracellular gram-negative bacteria that are transmitted by an arthropod vector, typically a tick. Their pathogenicity in people and dogs is well understood; in cats little is known currently. Ehrlichia organisms primarily infect leukocytes, while Anaplasma species typically infect erythrocytes, endothelial cells, platelets, as well as leukocytes. Reclassification of several rickettsial organisms within the families Rickettsiaceae and Anaplasmataceae (order Rickettsiales) occurred in 2001.40 The genera Ehrlichia was moved from the family Rickettsiaceae to the family Anaplasmataceae, while the genera Rickettsia remains in the family Rickettsiaceae.40 Within the genera Ehrlichia, E. phagocytophila, E. equi, and E. platys were moved to the genus Anaplasma, while E. risticii and E. sennetsu now belong to the genus Neorickettsia.40
Confirmation of rickettsial organisms as the causative agent of disease in cats is difficult. Rickettsia are difficult to culture, and morulae are infrequently present.35 In addition, the presence of morulae that are E. canis-like, for example, does not confirm infection with the specific Ehrlichia species because the morulae may belong to another species or genera. Serology has been used to diagnose rickettsial infections, but there are limitations. Serologic techniques among diagnostic laboratories are not standardized. Because there may be yet undiscovered rickettsial species targeting cats, serology results may be negative despite clinical disease. There is serologic cross reactivity among some rickettsial organisms, making diagnosis of infection with a specific species difficult.
Although the most effective therapy for treatment of feline rickettsial diseases is unknown, the American College of Veterinary Internal Medicine recommends that suspect ehrlichial cases be managed with doxycycline at a dose of 10 mg/kg/day for 28 days.35 Treatment of other rickettsial infections with doxycycline is also appropriate.
Ehrlichiosis
Vectors for E. canis include the ticks Rhipicephalus sanguineus and Dermacentor variabilis, and clinical ehrlichiosis in dogs has been well recognized and understood for decades.40 Although the first evidence of naturally transmitted ehrlichiosis occurring in cats was described in 1986, our understanding of the disease in cats and which Ehrlichia species are infective to cats is incomplete.35 Evidence for feline ehrlichiosis includes cytologic identification of E. canis-like morulae on blood smears, positive E. canis serology, and PCR evidence of ehrlichial organism DNA in blood.35
Feline ehrlichiosis has been recognized globally, because blood from five cats in North America and France was positive for DNA most consistent with E. canis.35 In addition, Ehrlichia-like morulae have been detected in peripheral leukocytes of cats in the United States, Kenya, France, Brazil, Sweden, and Thailand.5,35 Serology has been used as a diagnostic tool for evaluation of feline ehrlichiosis; however, a limitation is that seropositivity does not equate with active infection. There is a lack of standardization in available methodologies, and variable serologic cross reactivity occurs among species of Ehrlichia, Neorickettsia, and Anaplasma.35 Some cats with presumed ehrlichiosis test negative for E. canis antibodies but positive for N. risticii. Antibodies for N. risticii and Ehrlichia have been detected cats from Maryland, Virginia, California, and Colorado.
The pathogenesis of feline ehrlichiosis is thought to be similar to that of ehrlichiosis in dogs.35 Clinical disease has been described in 55 cats with probable E. canis-morulae in mononuclear cells, E. canis-like DNA in blood, or seropositivity for E. canis +/− N. risticii.35 Affected cats ranged from 1 to 14 years of age with no gender predisposition; most cats were domestic shorthairs5,35 Some cats had a history of tick infestation. Clinical signs included fever, anorexia, lethargy, weight loss, pallor, splenomegaly, lymphadenopathy, and anemia.35 Clinicopathologic abnormalities included anemia (both regenerative and nonregenerative), hyperglobulinemia, hypoalbuminemia, and positive antinuclear antibody titers. Both leukocytosis and leukopenia were documented.35 Some cats had radiographic evidence of interstitial lung disease.35 Concurrent infection with Mycoplasma haemofelis, M. haemominutum, Cryptococcus neoformans, feline immunodeficiency virus, or feline leukemia virus were documented.35
Cats with suspect ehrlichiosis have been treated with doxycycline, tetracycline, or imidocarb.35 In three cats, clinical resolution occurred with doxycycline therapy: 5 mg/kg PO q12h for 21 days. Five cats seropositive for N. risticii initially had clinical relapse after doxycycline therapy, but clinical resolution occurred after treatment with a higher dose: 10 mg/kg PO q12h for 21 days. Imidocarb dosed at 5 mg/kg IM administered as two injections 14 days apart was successful in treating two cats in Kenya.
Anaplasmosis
Anaplasma phagocytophilum is the causative agent of anaplasmosis in dogs and people, and there is evidence that cats can develop the disease after experimental inoculation as well as natural transmission. Ixodes tick species are vectors for transmission of A. phagocytophilum to dogs and are likely vectors for cats.2,34 At this point, it is not known if other modes of transmission, such as the ingestion of or contact with A. phagocytophilum–infected rodents, occurs in cats.34 In initial research studies, cats inoculated with A. phagocytophilum were found to have morulae in eosinophils but were asymptomatic.34 In a subsequent study, when cats with and without FIV infection were inoculated, they developed clinical disease.34
Other evidence for the susceptibility of cats to anaplasmosis includes the detection of A. phagocytophilum DNA in the blood of naturally infected cats in Sweden, Denmark, Ireland, and the United States. Additionally A. phagocytophilum–like morulae have been detected in neutrophils of infected cats in, Brazil, Kenya, and Italy.34 A. phagocytophilum morulae have been confirmed in the neutrophils of Swedish cats.34 Prevalence of A. phagocytophilum antibodies in 416 cats from six states in the United States was 4.3%, but blood samples were PCR negative for DNA from Anaplasma and Ehrlichia species.2 In Florida, 553 cats were tested for A. phagocytophilum by PCR and all were negative.2 At this time, it is not known if the prevalence of anaplasmosis is rare in cats or underdiagnosed because of limitations of current diagnostic tests.
The pathogenesis of feline anaplasmosis is likely similar to that in other species. The clinical manifestations of anaplasmosis in six cats diagnosed with infection, based on PCR documentation of A. phagocytophilum DNA with or without serologic evidence have been described.34 Cats were 9 to 14 months of age, and both castrated males and spayed females were infected.34 Cases occurred in Massachusetts, Connecticut, and Sweden.34 Clinical abnormalities were most often mild and included fever, lethargy, anorexia, tachypnea, and the presence of Ixodes tick.34
Clinicopathologic abnormalities included thrombocytopenia, neutrophilia with left shift, lymphopenia, and mild hyperglycemia. All cats were FIV and FeLV negative. Morulae were detected in only one cat; 24% of its neutrophils were affected. Of the three cats in which A. phagocytophilum serology was performed at presentation, two were seronegative and the third had a titer of greater than 1 : 640. Subsequently, the seronegative cats seroconverted, illustrating that negative serology at the time of initial clinical illness does not rule out anaplasmosis in cats. Titers for A. phagocytophilum increased, decreased, or fluctuated over time, so use of serology to confirm resolution of infection is not recommended. With treatment, five of the six cats became PCR negative within 15 to 139 days after diagnosis.34 All cats were seronegative for E. canis.34 Clinical disease in these 6 cats was milder than anaplasmosis in dogs; data from one study of cats experimentally co-infected with FIV and A. phagocytophilum suggests that immunocompromised cats may have more severe clinical disease.15
Rickettsia felis
The cat flea, Ctenocephalides felis is a reservoir and vector for R. felis, which is widely disseminated within tissues of the cat flea.1,44 Naturally infected C. felis fleas have been found worldwide, although prevalence of infection based on detection of R. felis DNA using PCR varies.8,28,44,52 In Italy, prevalence of R. felis in 320 cat fleas from 117 animals was 11.9%, while the prevalence was 9% in Germany.8,18 In one study in the United States, the prevalence of R. felis DNA in 226 cat fleas from 103 animals was 9%, while in another study 67% of cat fleas collected from cats from Alabama, Maryland, and Texas were positive for R. felis.1,28 R. felis–infected fleas have also been found in California, Florida, Georgia, Louisiana, New York, North Carolina, Oklahoma, and Tennessee. Rickettsia felis DNA has been found in two research cats exposed to fleas infected with R. felis.28 Most cats exposed to R. felis–infected fleas do not develop antibodies. This data suggest that R. felis may not cause clinical disease in cats, bacteremia may be brief or intermittent, or the organism is harbored in tissues so that blood samples tested by PCR are negative.
Cats may be potential reservoirs for Rickettsia felis and a source of infection in people. The pathogenicity of Rickettsia felis in cats is poorly understood. Cats experimentally infected remained asymptomatic but seroconverted between 2 to 4 months.23
1 Bayliss DB, Morris AK, Horta MC, et al. Prevalence of Rickettsia species antibodies and Rickettsia species DNA in the blood of cats with and without fever. J Feline Med Surg. 2009;11:266.
2 Billeter SA, Spencer JA, Griffin B, et al. Prevalence of Anaplasma phagocytophilum in domestic felines in the United States. Vet Parasitol. 2007;147:194.
3 Blondin N, Baumgardner DJ, Moore GE, et al. Blastomycosis in indoor cats: suburban Chicago, Illinois, USA. Mycopathologia. 2007;163:59.
4 Breider MA, Walker TL, Legendre AM, et al. Blastomycosis in cats: five cases (1979-1986). J Am Vet Med Assoc. 1988;193:570.
5 Breitschwerdt EB, Abrams-Ogg AC, Lappin MR, et al. Molecular evidence supporting Ehrlichia canis-like infection in cats. J Vet Intern Med. 2002;16:642.
6 Bromel C, Sykes JE. Epidemiology, diagnosis, and treatment of blastomycosis in dogs and cats. Clin Tech Small Anim Pract. 2005;20:233.
7 Bromel C, Sykes JE. Histoplasmosis in dogs and cats. Clin Tech Small Anim Pract. 2005;20:227.
8 Capelli G, Montarsi F, Porcellato E, et al. Occurrence of Rickettsia felis in dog and cat fleas (Ctenocephalides felis) from Italy. Parasit Vectors. 2009;2(Suppl 1):S8.
9 Crothers SL, White SD, Ihrke PJ, et al. Sporotrichosis: a retrospective evaluation of 23 cases seen in northern California (1987-2007). Vet Dermatol. 2009;20:249.
10 Davies C, Troy GC. Deep mycotic infections in cats. J Am Anim Hosp Assoc. 1996;32:380.
11 de Lima Barros MB, Schubach TM, Galhardo MC, et al. Sporotrichosis: an emergent zoonosis in Rio de Janeiro. Mem Inst Oswaldo Cruz. 2001;96:777.
12 Duncan C, Stephen C, Campbell J. Clinical characteristics and predictors of mortality for Cryptococcus gattii infection in dogs and cats of southwestern British Columbia. Can Vet J. 2006;47:993.
13 Duncan C, Stephen C, Lester S, et al. Follow-up study of dogs and cats with asymptomatic Cryptococcus gattii infection or nasal colonization. Med Mycol. 2005;43:663.
14 Duncan CG, Stephen C, Campbell J. Evaluation of risk factors for Cryptococcus gattii infection in dogs and cats. J Am Vet Med Assoc. 2006;228:377.
15 Foley JE, Leutenegger CM, Dumler JS, et al. Evidence for modulated immune response to Anaplasma phagocytophila sensu lato in cats with FIV-induced immunosuppression. Comp Immunol Microbiol Infect Dis. 2003;26:103.
16 Gaidici A, Saubolle MA. Transmission of coccidioidomycosis to a human via a cat bite. J Clin Microbiol. 2009;47:505.
17 Gerds-Grogan S, Dayrell-Hart B. Feline cryptococcosis: a retrospective evaluation. J Am Anim Hosp Assoc. 1997;33:118.
18 Gilles J, Just FT, Silaghi C, et al. Rickettsia felis in fleas, Germany. Emerg Infect Dis. 2008;14:1294.
19 Gilor C, Graves TK, Barger AM, et al. Clinical aspects of natural infection with Blastomyces dermatitidis in cats: 8 cases (1991-2005). J Am Vet Med Assoc. 2006;229:96.
20 Gionfriddo JR. Feline systemic fungal infections. Vet Clin North Am Small Anim Pract. 2000;30:1029.
21 Graupmann-Kuzma A, Valentine BA, Shubitz LF, et al. Coccidioidomycosis in dogs and cats: a review. J Am Anim Hosp Assoc. 2008;44:226.
22 Greene C. Histoplasmosis. In: Greene C, editor. Infectious diseases of the dog and cat. ed 3. St Louis: Elsevier; 2006:577.
23 Greene CG, Breitschwerdt EB. Cat-flea typhuslike illness (Rickettsia felis infection. In: Greene C, editor. Infectious diseases of the dog and cat. ed 3. St Louis: Elsevier; 2006:242.
24 Greene RT. Coccioidomycosis and paracoccidioidomycosis. In: Greene C, editor. Infectious diseases of the dog and cat. ed 3. St Louis: Elsevier; 2006:598.
25 Gremião IDF, Schubach TMP, Pereira SA, et al. Intralesional amphotericin B in a cat with refractory localised sporotrichosis. J Feline Med Surg. 2009;11:720.
26 Grooters AM, Taboada J. Update on antifungal therapy. Vet Clin North Am Small Anim Pract. 2003;33:749.
27 Harasen GL, Randall JW. Canine blastomycosis in southern Saskatchewan. Can Vet J. 1986;27:375.
28 Hawley JR, Shaw SE, Lappin MR. Prevalence of Rickettsia felis DNA in the blood of cats and their fleas in the United States. J Feline Med Surg. 2007;9:258.
29 Hodges RD, Legendre AM, Adams LG, et al. Itraconazole for the treatment of histoplasmosis in cats. J Vet Intern Med. 1994;8:409.
30 Johnson LR, Fry MM, Anez KL, et al. Histoplasmosis infection in two cats from California. J Am Anim Hosp Assoc. 2004;40:165.
31 Kano R, Kitagawat M, Oota S, et al. First case of feline systemic Cryptococcus albidus infection. Med Mycol. 2008;46:75.
32 Kerl ME. Update on canine and feline fungal diseases. Vet Clin North Am Small Anim Pract. 2003;33:721.
33 Lamm CG, Rizzi TE, Campbell GA, et al. Pathology in practice. Histoplasma capsulatum infections. J Am Vet Med Assoc. 2009;235:155.
34 Lappin MR, Bjoersdorff A, Breitschwerdt EB. Feline granulocytotropic anaplasmosis. In: Greene CG, editor. Infectious diseases of the dog and cat. ed 3. St Louis: Elsevier; 2006:227.
35 Lappin MR, Breitschwerdt EB. Feline mononuclear ehrlichiosis. In: Greene C, editor. Infectious diseases of the dog and cat. ed 3. St Louis: Elsevier; 2006:224.
36 Legendre AM. Blastomycosis. In: Greene C, editor. Infectious diseases of the dog and cat. ed 3. St Louis: Elsevier; 2006:569.
37 Malik R, Krockenberger M, O’Brien CR, et al. Cryptococcosis. In: Greene C, editor. Infectious diseases of the dog and cat. ed 3. St Louis: Elsevier; 2006:584.
38 Malik R, Martin P, Wigney DI, et al. Nasopharyngeal cryptococcosis. Aust Vet J. 1997;75:483.
39 Malik R, Wigney DI, Muir DB, et al. Cryptococcosis in cats: clinical and mycological assessment of 29 cases and evaluation of treatment using orally administered fluconazole. J Med Vet Mycol. 1992;30:133.
40 Neer MT, Harrus S. Canine monocytotropic ehrlichiosis and neoricketsisos (E. canis, E. chaffeensis, E. ruminatium, N. sennetsu, and N. risticii infections). In: Greene C, editor. Infectious diseases of the dog and cat. ed 3. St Louis: Elsevier; 2006:203.
41 O’Brien CR, Krockenberger MB, Martin P, et al. Long-term outcome of therapy for 59 cats and 11 dogs with cryptococcosis. Aust Vet J. 2006;84:384.
42 O’Brien CR, Krockenberger MB, Wigney DI, et al. Retrospective study of feline and canine cryptococcosis in Australia from 1981 to 2001: 195 cases. Med Mycol. 2004;42:449.
43 Odom T, Anderson JG. Proliferative gingival lesion in a cat with disseminated cryptococcosis. J Vet Dent. 2000;17:177.
44 Reif KE, Macaluso KR. Ecology of Rickettsia felis: a review. J Med Entomol. 2009;46:723.
45 Reis RS, Almeida-Paes R, Muniz Mde M, et al. Molecular characterisation of Sporothrix schenckii isolates from humans and cats involved in the sporotrichosis epidemic in Rio de Janeiro, Brazil. Mem Inst Oswaldo Cruz. 2009;104:769.
46 Schubach T, Schubach A, Okamoto T, et al. Evaluation of an epidemic of sporotrichosis in cats: 347 cases (1998-2001). J Am Vet Med Assoc. 2004;224:1623.
47 Schubach TM, de Oliveira Schubach A, dos Reis RS, et al. Sporothrix schenckii isolated from domestic cats with and without sporotrichosis in Rio de Janeiro, Brazil. Mycopathologia. 2002;153:83.
48 Schubach TM, Valle AC, Gutierrez-Galhardo MC, et al. Isolation of Sporothrix schenckii from the nails of domestic cats (Felis catus). Med Mycol. 2001;39:147.
49 Shubitz LF, Dial SM. Coccidioidomycosis: a diagnostic challenge. Clin Tech Small Anim Pract. 2005;20:220.
50 Spector D, Legendre AM, Wheat J, et al. Antigen and antibody testing for the diagnosis of blastomycosis in dogs. J Vet Intern Med. 2008;22:839.
51 Tofflemire K, Betbeze C. Three cases of feline ocular coccidioidomycosis: presentation, clinical features, diagnosis, and treatment. Vet Ophthalmol. 2010;13:166.
52 Tsai KH, Lu HY, Huang JH, et al. Rickettsia felis in cat fleas in Taiwan. Vector Borne Zoonotic Dis. 2009;9:561.
53 Vinayak A, Kerwin SC, Pool RR. Treatment of thoracolumbar spinal cord compression associated with Histoplasma capsulatum infection in a cat. J Am Vet Med Assoc. 2007;230:1018.
54 Welsh R. Sporotrichosis. J Am Vet Med Assoc. 2003;223:1123.
55 Yegneswaran PP, Sripathi H, Bairy I, et al. Zoonotic sporotrichosis of lymphocutaneous type in a man acquired from a domesticated feline source: report of a first case in southern Karnataka, India. Int J Dermatol. 2009;48:1198.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree