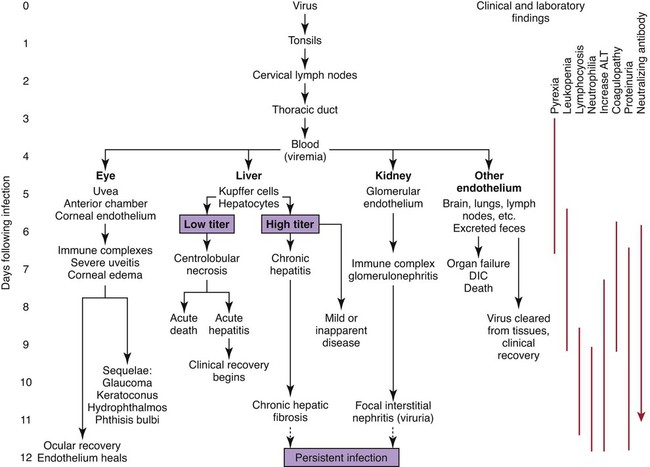
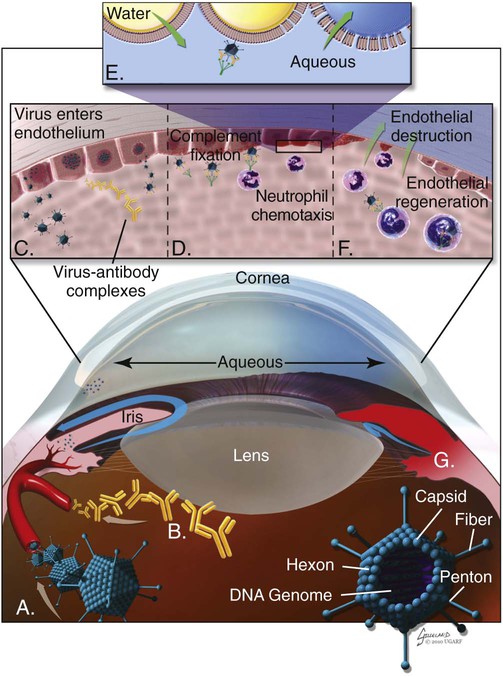
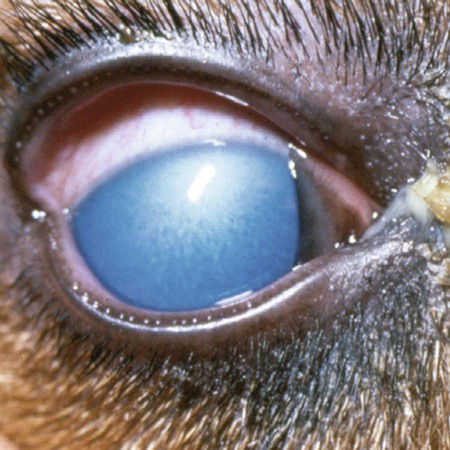
Infectious canine hepatitis (ICH), caused by canine adenovirus (CAV)-1, has worldwide serologic homogeneity, as well as immunologic similarities to human adenoviruses. Historical synonyms for the disease include fox encephalitis and Rubarth’s disease. CAV-1 is antigenically and genetically distinct from CAV-2, which produces respiratory disease in the dog (see Etiology, Chapter 6). Genetic variants of CAV-2 have been isolated from the intestine of a puppy with hemorrhagic diarrhea and from kenneled dogs with diarrhea. Human adenoviruses have been used as vectors for recombinant vaccine testing in dogs.26 CAV-1 causes clinical disease in dogs, coyotes, foxes, wolves, and other Canidae and in Ursidae (bears). A fatal infection was reported in an otter (Lutra lutra).31 In addition to these carnivores, serum antibodies have also been detected in marine mammals including walruses (Odobenus rosmarus) and sea lions (Eumetopias jubatus).5,32,32 The high prevalence of naturally occurring serum neutralizing antibodies in the unvaccinated feral and wildlife dog population suggests that subclinical infection is widespread.2,14,16,17,38 CAV-1 has been isolated from all body tissues and secretions of dogs during the acute stages of the disease. By 10 to 14 days postinoculation (PI), the virus can be found only in the kidneys and is excreted in the urine for at least 6 to 9 months. Aerosol transmission of the virus via the urine is unlikely, given that susceptible dogs housed 6 inches apart from virus shedders do not become infected. Viral spread can occur by contact with fomites, including feeding utensils and hands. Ectoparasites can harbor CAV-1 and may be involved in the natural transmission of the disease. After natural oronasal exposure, the virus initially localizes in the tonsils (Web Fig. 4-1), where it spreads to regional lymph nodes and lymphatics before reaching the blood through the thoracic duct. Viremia, which lasts 4 to 8 days postinfection, results in rapid dissemination of the virus to other tissues and body secretions, including saliva, urine, and feces. Hepatic parenchymal cells and vascular endothelial cells of many tissues including the central nervous system (CNS) are prime targets of viral localization and injury. Severe anterior uveitis and corneal edema develop 7 days postinfection, a period corresponding to an increase in neutralizing antibody titer (Fig. 4-1). CIC deposition with complement fixation results in chemotaxis of inflammatory cells into the anterior chamber and extensive corneal endothelial damage. Disruption of the intact corneal endothelium, which serves to pump fluid from the cornea into the anterior chamber, causes accumulation of edematous fluid within the corneal stroma. Abnormal physical findings in the early phase of infection include increased rectal temperature (39.4° C to 41.1° C [103° F to 106° F]) and accelerated pulse and respiratory rates. Fever may be transient or biphasic early in the course of the disease. Tonsillar enlargement, usually associated with pharyngitis and laryngitis, is common. Coughing and auscultated harsh lower respiratory sounds are manifestations of pneumonia. Cervical lymphadenomegaly is frequently found with subcutaneous edema of the head, neck, and dependent portions of the trunk. Abdominal tenderness and hepatomegaly are usually apparent in the acutely ill dog. A hemorrhagic diathesis that is demonstrated by widespread petechial and ecchymotic hemorrhages, epistaxis, and bleeding from venipuncture sites can occur. Icterus is uncommon in acute ICH, but it is found in some dogs that survive the acute fulminant phase of the disease. Abdominal distention is caused by accumulation of serosanguineous fluid or hemorrhage. CNS signs, including depression, disorientation, seizures, or terminal coma, can develop at any time after infection. In foxes and rare reports in domestic dog pups, CNS signs can be seen in the absence of other systemic manifestations.7 Mildly affected dogs may recover after the first febrile episode. Clinical signs of these uncomplicated cases of ICH frequently last 5 to 7 days before improvement. Persistent signs may be found in dogs with a concurrent viral infection such as canine distemper or in dogs that develop chronic active hepatitis. More severe or additional clinical signs occur in dogs that are co-infected with other pathogens.9,10,10 Corneal edema and anterior uveitis usually occur when clinical recovery begins and may be the only clinical abnormalities seen in dogs with inapparent infection (also see Infectious Canine Hepatitis in Chapter 92). Dogs with corneal edema show blepharospasm, photophobia, and serous ocular discharge. Clouding of the cornea usually begins at the limbus and spreads centrally (Fig. 4-2) (see Fig. 92-17). Ocular pain, present during the early stages of infection, usually subsides when the cornea becomes completely clouded. However, pain may return with the development of glaucoma or corneal ulceration and perforation. In uncomplicated cases, clearing of the cornea begins at the limbus and spreads centrally. Coagulation abnormalities characteristic of DIC are most pronounced during the viremic stages of the disease.30 Thrombocytopenia with or without altered platelet function is usually apparent. One-stage prothrombin time, activated partial prothrombin time (aPTT), and thrombin time are variably prolonged. Early prolongation of the aPTT probably results from factor VIII consumption. Factor VIII activity is decreased, and fibrin or fibrinogen degradation products are increased. Platelet dysfunction and later prolongation of the aPTT probably result from increased fibrinogen degradation products. Prolongation of the prothrombin time is usually less noticeable. Immunofluorescent techniques are used experimentally to confirm the presence of virus within various tissues. This method has helped locate the sites of viral replication, the spread of the virus within the cells, and the presence of viral antigen in inclusion bodies. Immunoperoxidase procedures, applied to formalin-fixed, paraffin-embedded tissues, have detected virus in liver tissues stored for up to 6 years. Polymerase chain reaction (PCR) techniques have been developed to detect CAV-1 in biologic specimens and to distinguish the virus from CAV-2 in clinical specimens.8,20,20
Infectious Canine Hepatitis and Canine Acidophil Cell Hepatitis
Infectious Canine Hepatitis
Etiology
Pathogenesis
Clinical Findings
Diagnosis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Veterian Key
Fastest Veterinary Medicine Insight Engine