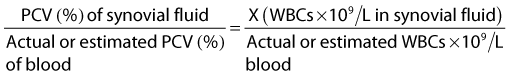Chapter 65Infectious Arthritis and Fungal Infectious Arthritis
 Infectious Arthritis
Infectious Arthritis
Diagnosis
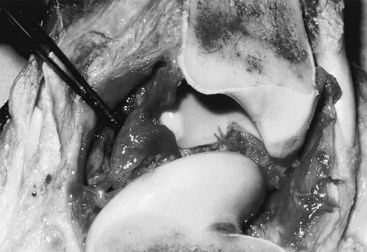
Gross evaluation of the synovial fluid can be informative. If newspaper print cannot be read through the fluid sample, it probably has a cell count of at least 30 × 109 nucleated cells per liter. Fluid from infected joints is usually turbid, cloudy, and watery. Flocculence develops in chronically infected joints or joints that have been invaded with a needle or surgery. Blood contamination makes gross assessment of synovial fluid difficult, and determining if infection is present without clinicopathological analysis is often impossible. Serosanguineous fluid commonly is obtained from infected joints (Figure 65-1). An estimate of the amount of blood (packed cell volume [PCV)] or red blood cell count) can be made to correct for the number of nucleated cells contaminating the sample (hemorrhage from arthrocentesis or leakage of blood from the infection):
where WBCs are white blood cells.
Nuclear scintigraphy may be useful to locate foci of infected bone, particularly in identifying involvement of subclinical sites of infection in polyarthritic foals, but the technique has practical restrictions. Normal bone scans or radiolabeled white cell studies can be performed (see Chapter 19). The foal becomes radioactive, and handling of the foal, blood samples, and synovial fluid samples poses a small risk to attendants. In addition, if the joints surrounding the small bones are infected, the resolution of the scan may be inadequate to identify specific bone involvement. Magnetic resonance imaging offers promise of improved identification of osteomyelitis31,32 and has become available at many referral hospitals. Osteomyelitis is present in up to 59% of foals with infectious arthritis, but a favorable prognosis still can be achieved with treatment,1 although the prognosis for athletic function is reduced when adjacent bone is involved or infectious arthritis is protracted.