CHAPTER 5 Infections of Muscle, Joint, and Bone
Musculoskeletal infections are a common clinical problem encountered in equine practice. Infections in the adult horse are often associated with trauma. Conversely, musculoskeletal infections in the foal are more likely to be of hematogenous origin. In both foals and adults, musculoskeletal infections are associated with significant morbidity and mortality. A rapid, accurate diagnosis and prompt initiation of appropriate therapy are important for a successful outcome.
MUSCLE INFECTIONS
Primary Bacterial Myositis
Etiology
Bacterial infection occurring in association with trauma is the most common form of primary infectious myositis. Streptococcus equi subsp. equi (see Chapter 28), Clostridium spp. (see Chapter 45), and Staphylococcus spp. (see Chapter 29) are common bacterial isolates from these cases.1 Mixed gram-negative and anaerobic bacterial muscle infection with abscessation can occur secondary to infection of deeper structures.1,2 Many different bacterial agents can colonize muscle from a hematogenous route, including S. equi subsp. equi and Clostridium spp.3–6 Salmonella can cause localized myonecrosis and infection secondary to septicemia in both adult horses and foals7 (see Chapter 38).
Diagnosis
For localized infection, ultrasound evaluation, radiography, and scintigraphy may assist diagnosis and interventional strategies. Even with an obvious abscess, ultrasound of affected muscle can facilitate evaluation of the extent of the lesion and assessment of response to therapy. Radiographs of the affected area may be indicated if skeletal involvement is suspected. Nuclear scintigraphy has been suggested to aid in localization of abscesses in muscle either by soft tissue phase imaging or by the use of radiolabeled autologous white blood cells.8
Diagnostic testing should include efforts to isolate or identify the causative agent. For localized infections, aspiration and culture of soft or fluid-filled swellings is recommended. Culture of samples obtained by deep swab of draining tracts is important. Both these techniques should be performed aseptically. Culture of fine-needle aspirates from swollen, inflamed muscles may yield inciting organisms such as S. aureus and C. pseudotuberculosis. Muscle biopsy may be indicated in some cases to confirm the presence of myositis and obtain diagnostic culture results. Aerobic, anaerobic, and fungal cultures should be requested when indicated. Identification of parasites is usually accomplished with histopathology. Serology can aid diagnosis of C. pseudotuberculosis (see Chapter 30). High S. equi subsp. equi titer may reflect recent exposure or active infection, if vaccination has not been recent.
Clostridial Myonecrosis
Etiology
Clostridial myonecrosis, gas gangrene, and malignant edema are all terms used to describe a rapidly progressing infection of muscle with Clostridium spp. resulting in severe myonecrosis9 (see Chapter 45). Clostridial myonecrosis occurs most often after intramuscular (IM) or perivascular injections but may also be associated with traumatic puncture wounds. It may occur in horses after deep IM injection of a variety of substances, including flunixin meglumine, ivermectin, B-complex vitamins, vitamin E, selenium, synthetic prostaglandins, dipyrone, phenylbutazone, antihistamines, and vaccinations.9,10 Clostridium septicum and C. perfringens are the species most often cultured from areas of myonecrosis in horses; however, other Clostridium spp., including C. chauvoei, C. novyi, and C. fallax, have also been isolated from affected horses.
The origin of the bacteria is unknown. Attempts to culture clostridial organisms from external sources have been unsuccessful. Some authors have hypothesized that spores are present in normal muscle, and that colonization occurs after IM injection with an irritating substance provides a suitable anaerobic environment for bacterial proliferation. Clostridial exotoxins play a central role in the massive myonecrosis associated with infection. These exotoxins directly affect the venous endothelium, creating intravascular platelet aggregation and leukostasis. Resultant decreases in tissue pH and oxygen tension impair immune defenses and create an ideal environment for clostridial growth.11 Clostridial exotoxins can also have a significant systemic effect, causing shock and in some cases hemolytic anemia.12,13
Clinical Findings
Clinical signs of clostridial myonecrosis typically develop within 6 to 72 hours of IM injection.10,14 Affected horses often show signs of shock and are painful, tachycardic, and dehydrated. It is not unusual for horses with clostridial myonecrosis to present for colic. Horses with cervical muscle infections may be lame in a thoracic limb and may have severe facial swelling. Palpable subcutaneous crepitus is considered a hallmark of this disease but may be absent early in the disease, especially if deep (gluteal) muscles are involved. Many horses will have localized swelling, heat, and sensitivity at the injection site; as the disease progresses, however, the skin may become cool and discolored. Any anatomic location used for IM injection can be affected; the cranial cervical muscle region may be affected in association with inadvertent perivascular injections. Clinicopathologic abnormalities are variable and may be consistent with shock. Increases in serum muscle enzyme activities may be seen, but rarely reflect the severity of the condition, most likely because of poor perfusion of the necrotic area.15
Historically, the prognosis for horses with clostridial myonecrosis has been considered to be guarded to poor.9 More recently, a retrospective study of 37 horses with clostridial myonecrosis reported an overall survival rate of 73%.10 Several factors appear to be important in the successful management of this disease. First, prompt diagnosis and early initiation of antimicrobial therapy (typically high doses of penicillin G) are very important. Second, the early use of fasciotomy and myotomy appears to improve prognosis. Both retrospective studies report a better prognosis with C. perfringens infections (19%-25% mortality) than with infection by other clostridial organisms, especially C. septicum (50%-85.7% mortality).10
Diagnosis
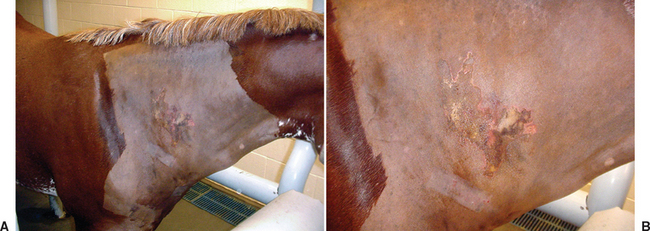
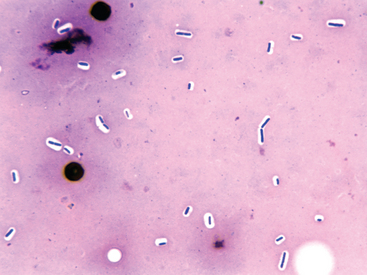
A history of recent IM injection with physical examination findings consistent with severe acute injection site infection is sufficient for a presumptive diagnosis of clostridial myonecrosis (Fig. 5-1). Ultrasound of the affected tissues may support the diagnosis, revealing fluid accumulation, gas, and changes in the muscular/fascial echotexture. Fluid aspirate cytology or muscle sample impression smear with Gram staining can immediately confirm the presence of numerous gram-positive rods characteristic of clostridial infection (Fig. 5-2). Fluid and/or muscle samples should be submitted for anaerobic cultures.
Therapy
Prompt antimicrobial therapy is necessary for effective treatment of clostridial myonecrosis. High doses of potassium penicillin G, 44,000 IU/kg body weight intravenously (IV) every 4 to 6 hours (q4-6h), are usually recommended. However, clostridial organisms are generally susceptible to a number of antibiotics, including oxytetracycline, chloramphenicol, metronidazole, and rifampin. Evidence in a mouse model of C. perfringens myositis suggests that oxytetracycline, metronidazole, and rifampin therapy may be more effective than treatment with potassium penicillin G.16 This is likely related to the ability of these antimicrobials to decrease toxin production. The ideal antimicrobial regimen would have potent bactericidal effects and decrease toxin production. In vitro, C. perfringens α-toxin production and survivability are high with penicillin G treatment, suggesting that this may not be the optimal antimicrobial choice. Tetracycline and chloramphenicol suppress toxin production but have limited effect on survivability. Rifampin and metronidazole have superior efficacy for suppression of both survivability and toxin production.16 Therefore, combination therapy with penicillin G and either rifampin or metronidazole may be appropriate. However, a subsequent study using the mouse model of C. perfringens myositis showed decreased survivability with penicillin G and metronidazole combination treatment compared with metronidazole alone.16 Currently, equine clinical data suggest that high doses of penicillin G are the first antibiotic choice for treatment of horses with clostridial myonecrosis.10
Myotomy and fasciotomy are important in the treatment of clostridial myonecrosis. These procedures allow drainage and debridement of necrotic areas, oxygenation of tissues, and some degree of disinfection. In at least one large retrospective study, myotomy and fasciotomy performed early in the course of disease were thought to result in decreased overall mortality.10 These authors suggest that myotomy and fasciotomy should be performed as soon as possible, stressing the importance of performing a Gram stain on wound exudate at that time. Although there are no guidelines as to where and how many myotomy and fasciotomy incisions to make, starting in areas with palpable subcutaneous crepitus or severe muscle damage is prudent (Fig. 5-3).
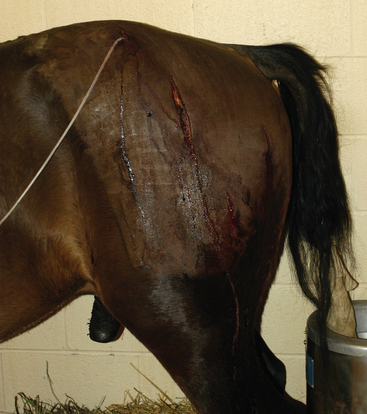
Fig. 5-3 Horse with multiple small fasciotomies and oxygen insufflation through proximal fasciotomy.
Hyperbaric oxygen, if available, may be beneficial for treatment of horses with clostridial myonecrosis but should be used in conjunction with standard treatment protocols. In combination with penicillin G or metronidazole in mice, early hyperbaric oxygen treatment resulted in increased survival compared to treatment with antimicrobial therapy alone.17
Primary Fungal Myositis
Etiology
Primary fungal myositis may occur after direct inoculation of the etiologic agent, as may occur with phycomycoses (see Chapter 55). Alternatively, hematogenous dissemination of systemic fungi may result in infectious myositis18,19 (see Chapter 51).
Clinical Findings
Most fungal infections are localized and fairly obvious. Coccidioidomycosis may be localized or may have concomitant widespread systemic disease with infection of the lung and liver (see Chapter 51).
Primary Parasitic Myositis
Etiology
The most common causes of parasitic myositis include Sarcocystis spp.,1,20,21 Trichinella spp.,22–24 and Trypanosoma evansi.25 Aberrant parasitic migrations from various nematodes can occur. Rarely, hydatidosis has been associated with infectious myositis.26
Diagnosis and Therapy
Diagnosis of parasitic myositis is usually accomplished by biopsy and histopathologic examination of the affected muscle. Treatment of specific parasitic infections is discussed elsewhere in this text. The most important health risk for humans associated with equine muscle infection is related to consumption of parasitized horse meat.21,23,27–31 Outbreaks of human trichinellosis occur fairly regularly in the countries of the European Union. Horse meat is screened for infected muscle in slaughter plants; however, it is advisable that preparation of human meals with horse meat follow appropriate guidelines for inactivation of Trichinella larvae in equine muscle before consumption.
Secondary Infectious Myositis
Etiology
Streptococcal infections and several viruses may contribute to development of secondary myositis in which viable infectious agents are not present in the affected muscle tissue.3–6 Lesions are usually generalized, affecting several muscle groups. The primary infectious agent triggers myositis as an inflammatory or an immune-mediated process. Streptococcus equi subsp. equi is associated with two types of myositis in horses: acute severe myositis, characterized by infarction of muscle, and chronic generalized muscle wasting. Both manifestations of streptococcal myositis are thought to be immune-mediated disorders and are discussed in more detail in Chapter 28. Myositis may occur concomitant with, or as a sequela to, acute viral infection. Viral myositis has been demonstrated or postulated to occur secondary to infection with equine herpesvirus (see Chapter 13), equine influenza virus (see Chapter 12), and African horse sickness (see Chapter 15).
Clinical Findings
Horses with myositis secondary to systemic infection present with generalized stiffness and lameness. Most affected horses demonstrate reluctance to move, and laminitis is the most common differential diagnosis. Muscles may or may not be painful on palpation. Horses with muscle infarction or necrosis may have areas of localized edema and pain. When widespread, these horses can have signs of circulatory failure accompanied by poor peripheral perfusion.32,33
Diagnosis and Therapy
Diagnosis of secondary myositis is accomplished by muscle biopsy to demonstrate histopathologic lesions of immune-mediated or inflammatory myositis. The approach to diagnosis of a specific underlying systemic infection depends on the type of infection that is suspected. Diagnosis of S. equi subsp. equi infection is discussed in Chapter 28. Diagnosis of equine influenza, equine herpesvirus, and African horse sickness is discussed in Chapters 12, 13, and 15, respectively.
SYNOVIAL INFECTIONS
Septic arthritis and tenosynovitis are common clinical problems in horses with potentially devastating consequences. Mortality estimates vary between 15% and 50%.34–38 In adult horses, these infections are most often caused by direct bacterial contamination of a synovial structure resulting from trauma or as a sequela to surgery or intrathecal injection. Hematogenous spread of infection is much rarer in adult horses but should not be overlooked as a differential diagnosis in the acutely lame horse. In foals, most synovial infections are of hematogenous origin. Failure of transfer of passive immunity, respiratory infection, and gastrointestinal infection should be considered as potential concurrent problems in foals diagnosed with septic synovial structures (see Chapter 6).
Etiology and Pathogenesis
A variety of bacteria may be isolated from synovial infections that are traumatic in origin. Enterobacteriaceae and anerobes are most frequently isolated. Horses that develop infection as a sequela to surgery or intrathecal injection are more likely to have staphylococcal infections.34
Historically, synovial infections in foals were postulated to originate from umbilical infections. It is now recognized that these infections may originate from other sources, including the respiratory and gastrointestinal tracts. Foals with poor transfer of passive immunity are predisposed to septic arthritis.39 The pathogens most frequently isolated from foals with neonatal sepsis are also the bacteria most often isolated from joints of foals with septic arthritis (see Chapter 6). Young foals (<3 weeks) are more likely to have infection of multiple joints, whereas older foals (>4 weeks) generally have only one affected joint.40 The most common bacterial organisms isolated from septic arthritis in foals are Enterobacteriaceae, most notably Escherichia coli. Other gram-negative organisms, such as Salmonella spp., are relatively common isolates. The most common gram-positive organisms isolated from foals with septic arthritis include Staphylococcus, Streptococcus, and Rhodococcus equi.34
Fungal infections of synovial structures are rare but should be considered, especially if a fungal organism is cultured from more than one site or more than one sample from the same site. Fungal infection of synovial structures may originate either hematogenously or by direct inoculation.41
Clinical Findings
The hallmark clinical sign of synovial infection in the appendicular skeleton of the horse is severe lameness. The onset and severity of clinical signs depend on the mode of contamination, degree of contamination, pathogenicity of the organism involved, amount of open drainage from the synovial structure, and previous treatment with intraarticular corticosteroids. Although clinical signs are evident within 24 hours of experimental inoculation of equine joints with bacteria,42,43 the onset of clinical signs from “natural” infection appears to be slower. In one study, joint infections on average became apparent on day 8 after surgery, with a range of 1 to 25 days.34 It is questionable whether all affected joints were inoculated at surgery.
The onset of clinical signs of synovial infection after trauma is variable. Much of this variability may be explained by the degree of open drainage (horses with sealed synovial infections tend to be more lame), inflammation, and pain associated with tissue trauma, which may be indistinguishable from that caused by synovial infection, and by delayed recognition by owners or trainers. The onset of lameness and clinical signs of synovial infection after intrathecal injections is also somewhat variable, depending on the factors just listed as well as whether or not corticosteroids were administered. Tulamo et al.43 showed that co-administration of corticosteroids with an infective dose of bacteria significantly delayed clinical signs and synovial fluid changes for up to 2 days. In two retrospective studies describing infection after intrathecal injection, the onset of clinical signs varied from 2.5 to 7 days.34,38
Synovial effusion, local edema, heat, and sensitivity to palpation are usually observed in horses with synovial infection. Experimental models of infection suggest that these signs may briefly precede clinical lameness.42,43 Body temperature is usually increased in synovial sepsis,43 but the lack of a fever does not rule out joint sepsis, especially in adult horses. Foals typically have higher fevers than adult horses. In one study, 45% of foals with septic arthritis had a body temperature 102° F (38.9° C) or higher.34
Diagnosis
Synovial Fluid Analysis
Synovial fluid analysis is necessary for the diagnosis of infection. Grossly, synovial fluid from an infected joint is serosanguineous and turbid (increased cellularity) with decreased viscosity resulting from decreased hyaluronic acid content. Samples from affected joints may contain visible fibrin and debris. The sample should be submitted for total and differential cell count, total protein measurement, and immediate culture. Most samples from infected synovial structures have a total white blood cell (WBC) count greater than 30,000 cells/μL (normal <1000/μL, predominantly mononuclear cells), a differential with 90% or more neutrophils, and a total protein concentration greater than 4.0 g/dL (normal <2.0 g/dL). A Gram stain should be performed; positive findings confirm the diagnosis and may guide antimicrobial therapy. A sample of synovial fluid should be cultured aerobically and anaerobically. The fluid should be cultured in a broth culture system designed for culturing body fluids44 (see Chapter 27). Synovial biopsy and culture do not yield better results than culture of synovial fluid and are not recommended.45