, Monika Hassel2 and Maura Grealy3
(1)
Centre of Organismal Studies, University of Heidelberg, Heidelberg, Germany
(2)
Spezielle Zoologie, Universität Marburg FB Biologie, Marburg, Germany
(3)
Pharmacology and Therapeutics, National University of Ireland Galway, Galway, Ireland
8.1 Germ Line and Primordial Germ Cells
8.1.1 Primordial Germ Cells Are Set Aside Early in Development or Derive from Stem Cells Late in Development: Maternal “Germ Plasm Determinants” Versus Inductive Determination by the Somatic Environment
Frequently in embryo development cells which are not used to construct the soma – the body of the new individual – are put aside and remain in stock as primordial germ cells. They are spared from constructing the mortal body because their task is to transmit potentially immortal life in the germ line (Figs. 8.1 and 8.2) to the next generation before the soma, the individual’s body, dies.
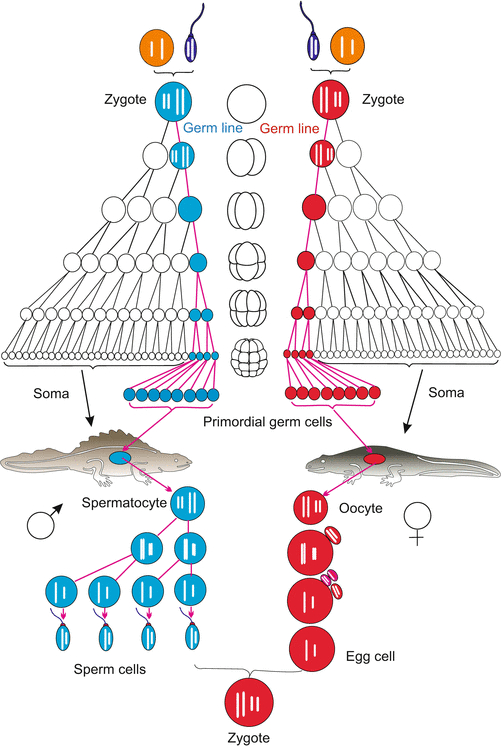
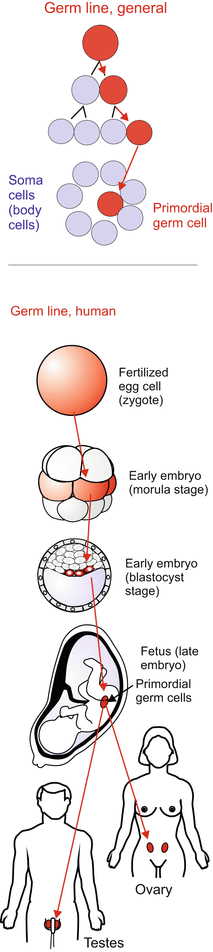

Fig. 8.1
Germ line (red or blue), shown using the example of great crested newt (Triturus cristatus)

Fig. 8.2
Germ line in humans
Early versus late commitment of primordial germ cells. Nowadays distinction is drawn between early predetermination dependent on maternal factors, collectively referred to as germ plasm, and late assignment to stem cells of germ cell fate by the somatic surroundings.
Extreme cases of early commitment are known from some nematodes and from Drosophila.
Nematodes. When observing the chromosomes in the cleaving embryo of the roundworm Ascaris (now, Parascaris equorum or Parascaris univalens) at the turn of the last century, Theodor Boveri (1862–1915) observed unusual behaviour in those chromosomes. This nematode has only two pairs of large compound chromosomes (i.e. several chromosomes sticking together). The first divisions are asymmetric (compare C. elegans, Figs. 4.20 and 9.7). After the first cleavage, the compound chromosomes in one of the two blastomeres fragment into many pieces and many of the fragments are lost. This phenomenon of chromatin diminution occurs in the cell destined to participate in the construction of the body. The eliminated chromatin predominantly contains repetitive sequences and a few genes that are required in the development of the germ cells but not in soma cells. The task of giving rise to germ cells is assigned to the second blastomere and in this cell the chromosomes remain intact. This second blastomere, designated P1, is the founder cell of the germ line. During the next divisions the story is repeated: only the cells on the direct route to the primordial germ cells preserve the entire chromosomes, whereas all future somatic cells lose chromatin. The P lineage leads to the primordial cell P4. This P4 cell is set aside and later in the adult worm used for producing germ cells. By experimental interference, such as centrifugation, Boveri was able to demonstrate that localized “cytoplasmic determinants” protect chromosomes of the germ line cells from being fragmented. Moreover, these cytoplasmic factors were thought to commit their host cells to become germ cells.
In the nematode Caenorhabditis elegans (Figs. 4.19 and 4.20) which displaced Ascaris as model organism, chromatin diminution is not known. Nevertheless also in the eggs of C. elegans such germ line-determining components exist embedded in granules. These are allocated to the P line (Figs. 8.3a and 4.20). These granules embody, among other components, mRNA of genes that are homologous to the Drosophila genes vasa, piwi and nanos. Their activity is distinctive of the germ line in animals in general as discussed below under Sect. 8.1.2.
In Drosophila the primordial germ cells are the first cells to be formed in the cleaving embryo. They are called pole cells because they arise at the posterior pole of the egg (Fig. 8.3b, also Figs. 4.26 and 4.30). As the pole cells form, they enclose pole granules similar to those seen in nematodes. These are fibrous conglomerates containing considerable protein and RNA, and embody germ cell determinants. If pole plasm is removed with a micropipette from the posterior egg pole and transferred into the anterior pole of another egg, oogonia or spermatogonia arise at this ectopic site (Fig. 4.20) (ectopic from Greek ek, ekto = outside, topos = place; outside the normal place). Not all of the determining components are identified as yet but it is established that the products of the maternal genes vasa, nanos and piwi are of particular significance. An unusual component of these granules is RNA of mitochondrial origin which has been released by mitochondria. But this mitochondrial RNA probably is not a decisive germ cell determinant itself. The determinants are allocated to the posterior region of the egg under the organizing influence of oskar. When injected beneath the anterior pole of the egg, the oskar product causes ectopic development of pole cells at the anterior end of the embryo. These, however, will not find their way into the gonads. Only pole cells properly located at the posterior pole are eventually incorporated into the gonads.
Amphibians. We discussed earlier the remarkable work that has made it possible to follow the developmental heritage of every cell in nematodes, but even in vertebrates the lineage of the primordial germ cells can be traced back to the uncleaved egg. In Xenopus, certain staining procedures demonstrate a particular “germ plasm” – granular cytoplasmic constituents close to the vegetal pole of the egg (Fig. 8.3c). Like the pole cytoplasm in Drosophila this region harbours RNA-loaded granules. Later these granules are found in the primordial germ cells that arise in the region of the embryonic hindgut and migrate along the mesenteries (devise to suspend the intestine) into the gonadal ridges (the rudiments of the ovaries or testes).
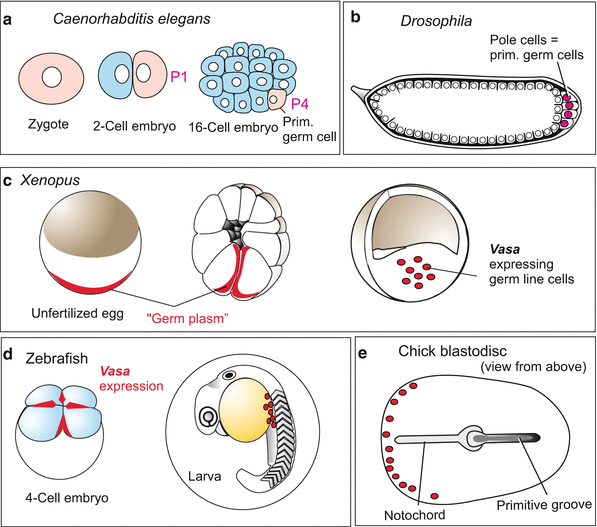
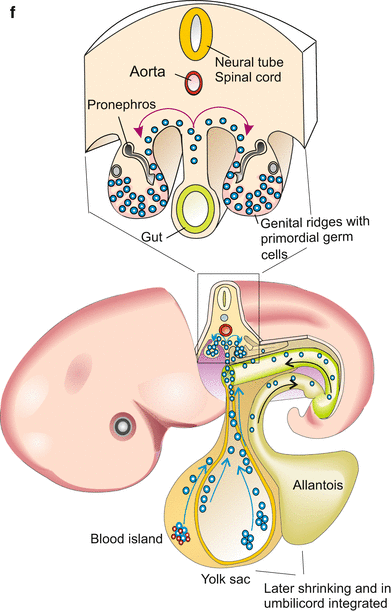
Fig. 8.3
(a–f) Origin of primordial germ cells in various animals. (a–e) The gene vasa is expressed in the germ line possibly of all animals. Sites where vasa products have been found are marked in red. (f) In mammals/humans traditional notions see the origin of the primordial germ cells in the allantois, recent reports find stem cells with the potency to give rise to germ cells in blood islands which are dispersed in the cover of the yolk sac
The term germ plasm (“Keimplasma”) was coined by the zoologist August Weismann at about 1893 but had a meaning very different from the meaning the term obtained in the subsequent literature, and has in the present literature. In Weismann’s terminology germ plasm is the entirety of all – in those days still unknown – material carriers of hereditary information. His “germ plasm” can rather be equalized with the term “genome” of our days. Presumably stimulated by Theodor Boveri’s observation on chromatin diminution in Ascaris Weismann assumed only the cells of the germ line (“Keimbahn”, his term also) would be equipped with the entire hereditary material, while soma cells would obtain only those parts required for, and being the cause of, differentiation.
Late determination:
Hydrozoa. Particularly late is the segregation of soma and germline in Hydractinia echinata. Here in the gonads a last asymmetric division of pluripotent precursors generate primordial germ cells and somatic envelope cells. Up to this point the germ line passes through the population of pluripotent interstitial stem cells which can give rise to somatic and generative cells in the mature animal at any time.
Vertebrates, birds. In the avian (and likewise the mammalian) embryo the first cells that can be identified as germ cell precursors are found in the extra-embryonic mesoderm at the site where the allantois, the embryonic precursor of the urinary bladder, begins to form. Subsequently germ cell precursors appear in the posterior region of the yolk sac (Fig. 8.3). Compared to amphibians this emergence of germ cell precursors appears to be a late event. Recent studies attribute commitment to inductive influences of the microenvironment, called a niche. Induced by these local influences some cells withdraw from companions in fate to enter the path to germ cells. Once committed, primordial germ cells set off to find the distant primordia of the gonads. Starting from their home place they take a similar route as in the amphibian embryo (see Fig. 15.2). In part they use, like the cells of the immune system, the blood stream to drift near to their destination. The destination sites send chemotactic signals (SDF-1-factor), to pinpoint the location of the destination.
Mammals appear to take an intermediate position between early specification and late commitment. An early though weak expression of germ line specific genes is already seen in the blastocyst. The first morphologically identifiable primordial germ cells are found in the blood islands dispersed over the yolk sac (Fig. 8.3f). They are descendants of pluripotent stem cells which also give rise to blood cells (Figs. 17.2 and 17.3). From here they emigrate, taking a similar route as in amphibians and birds, to reach the genital ridge where the gonads form. During migration expression of germ line specific genes is intensified.
8.1.2 Early Determination: Maternal Constituents, Referred to as “Germ Plasm” and a Complex Called “Nuage” Imprint Primordial Germ Cells
As stated above in C. elegans “P granules”, in Drosophila a “pole plasm” and in amphibians a “germ plasm” have been identified. In all these cases the particular plasm marks future germ cells. Similar types of “germ plasm”, also called “germinal granules”, are found in germ cell precursors of increasing numbers of other animal species as well.
The pole or germ plasm is an accumulation of granules that are not enclosed by membranes and contain dense aggregates of RNA and protein (RNP particles). This applies also to the P granules in the egg cell of C. elegans.
In amphibians, fishes and also invertebrates such as the Platynereis one finds, in addition to, or instead of, these granules, an accumulation of fibrous structures and mitochondria. Such aggregates have been described in the oocytes of more than 80 animal species and are referred to as nuage (from the French word for cloud). They harbour a large collection of enzymes and RNA species, especially DEAD box helicases, Tudor domain proteins and PIWI/argonaute proteins which bind piRNAs (piRNA = piwi-interacting RNA). In the ovary of the zebrafish 42,856 different piRNAs were found; they belong to the category of non-protein-coding, small RNA’s and in part derive from transposons.
Piwi proteins and piRNAs are also components of the chromatoid bodies CB in the spermatocytes and are considered to be generally germ line specific.
Germ plasm, nuage aggregates and CB particles contain, besides differing constituents, also identical gene products, for example VASA (a RNA helicase) and the transcription factor OCT-4. In mammals, no dense conspicuous granules attracted attention in the migrating germ cell precursors up to now, but cloudy and granular nuage-like material is described in oocytes and spermatocytes, and products of the genes VASA and Oct-4 are present.
Currently, the following functions are assigned to components of the germ plasm:
A leading part in committing cells to become germ cell precursors is assigned to the genes vasa and nanos .
Control of transcription and translation in the germ line cells is thought to be a main function of the piwi RNAs and proteins (piwi stands for P-element induced wimpy testis; in humans they are also called hiwi and in mice miwi). It is assumed that binding of small piRNAs onto messenger RNAs interrupts protein synthesis.
This translational control encompasses control of transposons in the germ line cells. In particular retrotransposons (see Chap. 12) need to be prevented from making mischief by jumping in the genome and disrupting genes.
Preservation of pluripotency in the germ line.
Commitment to germ cell fate and preservation of pluripotency are considered in more detail in the following section.
8.1.3 Products of the Genes Vasa, Nanos and Pluripotency-Conferring Genes Such as Oct-4 Make Cells of the Germ Line Primordial Germ Cells: Also in Humans
The pole plasm in the egg of Drosophila and the germ plasm in the embryo of Xenopus are known for decades. But not until 1999 a gene product promising to be a decisive component of the germ cell determinant was found. It is the RNA derived from the gene vasa. Homologues of this gene, initially identified in Drosophila, have been found in members of many animal phyla, in cnidarians, planarians, in the ecdysozoans Caenorhabditis elegans and Drosophila, in the annelid Platynereis and in chordates such as tunicates (Ascidia), the zebrafish Danio, the clawed frog Xenopus, the chicken, the mouse and humans. Defects in both alleles result in infertility.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


