Chapter 6 Immunohematology
Immune System
The immune system is an integrated network composed of various cell types, numerous cytokines, and certain plasma proteins that work in synergy to eliminate infectious agents, parasites, and noxious antigens. Consequently defects in the immune response result in increased susceptibility to these foreign invaders. However, inappropriate or exaggerated immune responses can result in immune-mediated tissue injury. Because a thorough review of immunology is beyond the scope of this text, the reader is referred to current immunology textbooks for more detailed information.140
Innate Immunity
The innate immune system responds immediately once an invading organism is detected, but it lacks any form of memory and responds in a similar manner and time frame to a repeated challenge by an invading organism. Innate immunity (nonspecific immunity) is possible because chemical compositions of invading microorganisms differ from those of normal body components. Innate immunity involves neutrophils, eosinophils, basophils, macrophages, mast cells, and natural killer (NK) cells, along with the complement system, enzymes such as lysozyme, and carbohydrate-binding proteins that can promote microbial destruction. These cells express microbial pattern-recognition receptors that recognize pathogen-associated molecular patterns (PAMP) on invading microorganisms. Following activation, the cells release components that can result in microbial destruction. Activated cells also produce various cytokines that result in inflammation and the recruitment of additional cells that can attack and destroy invaders. The production and function of these various cell types are discussed in Chapters 3 and 5, respectively.140
Acquired Immunity
Acquired immunity, also known as specific immunity or adaptive immunity, is a more recent evolutionary development than innate immunity. It is distinguished by its specificity for an invading organism and for its ability to remember an encounter with an invader so that a more rapid and intense response can occur the second time the same invader is encountered. Lymphocytes are immunocompetent cells that respond to specific foreign antigens. The production and function of lymphocyte types are discussed in Chapters 3 and 5, respectively. B lymphocytes are primarily responsible for immunoglobulin (antibody) production. In contrast to B lymphocytes, which produce immunoglobulins carried in the blood (humoral immunity) to the site of a foreign antigen, T lymphocytes migrate to the site of a foreign antigen (cellular immunity). T lymphocytes are involved in immune regulation, cytotoxicity, delayed-type hypersensitivity, and graft-versus-host reactions. Helper T (CD4+, CD8–) lymphocytes secrete cytokines that influence immune responses, and cytotoxic T (CD4–, CD8+) lymphocytes play pivotal roles in cell-mediated immunity directed at fungi, protozoan organisms, and neoplastic cells. Regulatory T lymphocytes function to maintain a balance between activation of the immune system and prevention of autoimmunity.30,81,140
Tests for Immune-Mediated Disorders
Tests for Antierythrocyte Antibodies
Direct Antiglobulin Test or Coombs’ Test
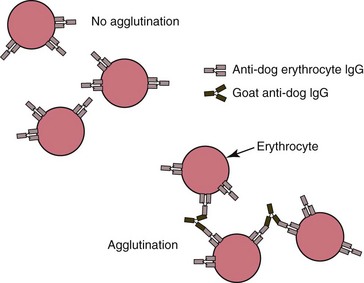
The direct antiglobulin test (DAT) utilizes washed erythrocytes from the patient and species-specific antisera against IgG, IgM, and the third component of complement (C3) to detect the presence of one or more of these factors on the surface of erythrocytes (Fig. 6-1). Blood should be collected in EDTA to avoid in vitro uptake of complement by erythrocytes.146 The DAT may be done in either tubes or microtiter plates.106 Unless clinical evidence of cold-agglutinin disease is present, this test is usually conducted only at 37oC, because a substantial number of healthy animals exhibit positive test results when the test is run at cold temperatures. In addition to primary immune-mediated hemolytic anemia (IMHA), neonatal isoerythrolysis, and blood transfusion reactions, the DAT may be positive in association with various infectious, parasitic, neoplastic, inflammatory, and other secondary immune-mediated diseases. If a drug-induced immune-mediated disorder is suspected, the offending drug should be included in the assay system.146
A negative DAT does not rule out an IMHA. A false-negative test may occur if there are insufficient quantities of antibody or complement on erythrocytes, the ratio of antiglobulin in the reagent to antibody or complement on erythrocytes is not appropriate, the test is performed with an incorrect species-specific reagent or at an improper temperature, the antibodies and/or complement elute from erythrocytes because the assay is delayed, the washing of erythrocytes is not adequate, the pH of the washing solution is too low, the centrifugation of the sample is not sufficient or there is excessive agitation in reading the tube test, or the drug was not added to the test for an animal with a drug-induced immune-mediated hemolytic anemia.146
False-positive tests may occur if clots are present (resulting in complement activation), blood is collected through infusion lines used to administer dextrose containing solutions, cryptantigens are exposed by the actions of bacterial enzymes on erythrocytes in septicemic patients, naturally occurring cold autoantibodies result in complement binding to erythrocytes, hypergammaglobulinemia is present, glassware or saline is contaminated, or if excessive centrifugation of tubes or misreading of results occurs.146
Direct Immunofluorescence Flow Cytometry Assay
Fluorescein isothiocyanate (FITC)-labeled antibodies against immunoglobulins of the species being evaluated are used to label erythrocyte-bound immunoglobulins, which are subsequently detected using flow cytometry. The direct immunofluorescence flow assay has greater sensitivity but somewhat lower specificity than the DAT assay when used to evaluate IMHA in dogs.121,146,155 The specificity is improved by setting a cutoff limit of greater than 5% positive cells before a test is considered positive. This should largely exclude low-level binding of immunoglobulin to normal (presumably aged) erythrocytes.104
Direct Enzyme-Linked Antiglobulin Test
The direct enzyme-linked antiglobulin test (DELAT) is an enzyme-linked immunosorbent assay (ELISA) that has been developed and evaluated for use in dogs. Regardless of the cause of the anemia, a majority of anemic dogs have increased erythrocyte-bound immunoglobulin and/or complement when the DELAT is used. This test has high sensitivity but low specificity for the diagnosis of primary IMHA. It is also time consuming and is typically used as a research tool and not in a clinical setting.7,146
Blood Typing
Large numbers of protein and complex carbohydrate antigens occur on the external surface of erythrocytes. Some antigens are present on erythrocytes from all members of a species and others (including blood group antigens) segregate genetically, appearing in some but not all members of a species. When an antigen is present in some members of the same species but is not common to all members of that species, it is called an alloantigen (also an isoantigen). If an alloantigen is presented to a member of the same species that does not have the alloantigen, it will be recognized as foreign and antibodies called alloantibodies (isoantibodies) will be produced against it.71
Blood group alloantigens are detected serologically on the surface of erythrocytes using agglutination and/or hemolysis tests. Blood groups have individual chromosomal loci and each locus has from two to many allelic genes. Most blood groups derive their antigenicity from the carbohydrate composition of membrane-associated glycolipids and glycoproteins. Most alloantigens are produced by erythroid cells, but some—such as the J group in cattle, the DEA-7 (Tr) group in dogs, the R group in sheep, and the A and O groups in pigs—are produced by other tissues and adsorbed from plasma.1,110
Blood groups in domestic animals have been reviewed.1,13,110 They have been most extensively characterized in horses and cattle, in which blood typing was routinely used for animal identification and parentage testing. Blood typing for these purposes is being phased out in favor of assays based on DNA sequences. Blood group alloantigens of clinical significance are discussed subsequently under “Transfusion Reactions” and “Neonatal Isoerythrolysis,” below.
Ideally, blood typing of donor and recipient animals for clinically significant erythrocyte alloantigens should be performed prior to all blood transfusions, as occurs in human medicine. Point-of-care card and gel typing tests are available for DEA 1.1 in dogs and types A and B in cats.141 In addition, blood samples from potential donors can be sent to a limited number of commercial laboratories for blood typing, and blood donors can be selected that are negative for clinically significant erythrocyte alloantigens, including DEA 1.1 in dogs and Aa and Qa in horses. The use of blood from these donors, coupled with cross-matching of donor and recipient samples, will minimize the likelihood of severe transfusion reactions.71
Blood typing of animals may be done prior to mating to identify animals with the same blood types and to minimize the possibility of subsequent hemolytic reactions (neonatal isoerythrolysis) in newborn animals. This is most frequently done in mares that have previously given birth to foals that developed neonatal isoerythrolysis. It may also be considered in certain breeds of cats where type B blood is common (Table 6-1).1,57,59,64
Table 6-1 Frequency of Blood Type B in Purebred Cats in the United Statesa
| Type B Frequency | Breeds |
|---|---|
| 25%-50% | Exotic shorthair, British shorthair, Cornish Rex, Devon Rex |
| 5%-25% | Abyssinian, Birman, Persian, Himalayan, Somali, Sphynx, Scottish fold, Japanese bobtail |
| Less than 5% | Main Coon cat, Norwegian forest cat, domestic shorthair, domestic longhair |
| None | Siamese, Burmese, Tonkinese, Russian Blue, Oriental shorthair, American shorthair, Ocicat |
a Type A frequency is determined by subtracting type B frequency from 100% because type AB is extremely rare. The table is modified from Andrews1 and based on data published by Urs Giger and coworkers.57,59,64
Blood Cross-Match Tests
Blood cross-match tests are used to detect the presence of hemagglutinating and hemolyzing antibodies in the serum of donor and recipient animals. Suspensions of washed erythrocytes are incubated with serum samples, centrifuged, and examined for the presence of hemolysis and gross and microscopic agglutination. The major cross-match is used to detect antibodies in the recipient’s serum that are directed against the donor’s erythrocytes. The minor cross-match is used to detect antibodies in the donor’s serum that are directed against the recipient’s erythrocytes. Autoagglutination or severe hemolysis in the patient’s blood sample precludes the accurate performance of cross-match tests.141
The absence of agglutination or hemolysis in cross-match tests does not indicate that animals have similar blood types. It indicates only that preexisting antibodies were not detected and that an acute hemolytic transfusion reaction is highly unlikely. A delayed transfusion reaction can still occur if important alloantigen differences are present. The benefit of the transfusion is short-lived in delayed transfusion reactions because antibodies made against the donor’s erythrocytes result in phagocytosis and removal of these erythrocytes within a few days.141 Additionally there can be reactions to transfused leukocyte or plasma protein antigens, with adverse reactions varying from urticaria to anaphylactic shock.77,157
Tests for Antinuclear Antibodies
The presence of circulating antinuclear antibodies (ANAs) is associated with various autoimmune diseases in humans and animals. ANAs are most often measured in dogs suspected of having systemic lupus erythematosus (SLE). Studies indicate that ANAs in dogs are primarily of the IgG type. Canine ANAs are heterogeneous and may be directed against various histone and nonhistone extractable antigen components of the nucleus but not against native double-stranded DNA.37
ANA Test
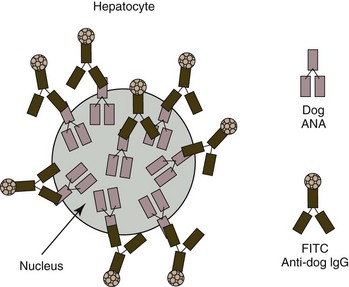
An indirect immunofluorescent antibody (IFA) technique is most widely used for ANA testing (Fig. 6-2). Typically a dilution of a patient’s serum is placed on a glass slide with tissue cells fixed to the surface. After allowing time for the ANAs present in the patient’s serum to become bound to the nuclei, the slides are rinsed and fluorescein-labeled antibodies directed against immunoglobulins of the same species as the patient’s are added. The slides are again rinsed and the absence or presence of nuclear fluorescence (which occurs when ANAs are present) is determined using a fluorescent microscope. Alternatively, an immunoperoxidase method may be used in place of the immunofluorescent one described. Frozen rodent liver sections have been used most frequently as the substrate in veterinary medicine, but a human epithelial cell line (HEp-2) appears to be a superior ANA substrate because of its low reactivity with normal serum and the ease of reading the fluorescence pattern. Titers above 1/25 and 1/100 are considered positive in dogs when HEp-2 and rat liver substrates, respectively, are used.67
Systemic autoimmune diseases are characterized by high serum ANA titers. This heterogeneous group of disorders may be subclassified as SLE or SLE-related diseases (called mixed connective tissue disease in humans). Two different nuclear staining patterns are recognized using HEp-2 cells as substrate in dogs. Dogs with homogeneous nuclear staining and positive chromosomal staining in mitotic cells are more likely to have SLE, and dogs with speckled nuclear staining and lack of chromosomal staining in mitotic cells are more likely to have SLE-related diseases.69
Propylthiouracil (PTU) treatment in cats can produce an immune-mediated disease syndrome characterized by anorexia, lymphadenopathy, weight loss, Coombs’-positive hemolytic anemia, thrombocytopenia, and a positive ANA serum test.3,115 Chronic experimental hydralazine treatment induced ANA formation in the serum of some Beagle dogs.4 The serum ANA test is positive in about one-third of Gordon setters with symmetrical lupoid onychodystrophy and black-hair follicular dysplasia, suggesting these may be immune-mediated disorders with a common genetic predisposition.107 ANAs may also be present in serum from animals with chronic inflammatory, infectious, and neoplastic diseases; however, titers are usually low. In addition, some healthy cats and dogs are weakly ANA-positive.12,37,60,108,136
Lupus Erythematosus Cell Test
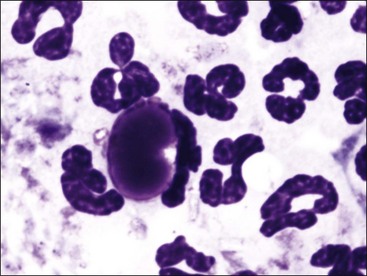
A lupus erythematosus (LE) cell is a leukocyte (usually a neutrophil) with a single large reddish-purple amorphous inclusion that nearly fills the cytoplasm of the cell (Fig. 6-3). This inclusion represents the nucleus of a damaged leukocyte that has been opsonized by ANA and complement and phagocytized by an intact leukocyte. LE cells occasionally form in vitro in stored anticoagulated blood, bone marrow, and joint fluids. The LE cell test is performed by promoting the formation of LE cells by rupturing leukocytes to expose their nuclear material either by forcing clotted blood through a sieve or by mixing anticoagulated blood vigorously with glass beads. After the leukocytes have been ruptured, the samples are incubated to allow time for LE cell formation. Buffy-coat smears are made, stained, and examined for the presence of LE cells. The finding of a single LE cell is considered a positive test result. With the ready availability of the ANA test, which is more sensitive and less labor-intensive to perform than the LE cell test, the latter test is now seldom done in veterinary laboratories. The advantage of the LE cell test is that it does not require species-specific reagents.37
Tests for Antiplatelet Antibodies
A number of tests have been developed to detect antiplatelet antibodies. These include a direct immunofluorescence test using labeled antibodies bound to megakaryocytes and various ways of detecting immunoglobulin bound to platelet surfaces. The microscopic detection of immunofluorescence of megakaryocytes is a subjective test requiring that a bone marrow aspirate be done to obtain megakaryocytes.90
Increased platelet-bound immunoglobulins can be detected by flow cytometry,84,100,156 immunoradiometric,132 ELISA,92 and microscopic platelet immunofluorescence asssays.87 Most antiplatelet antibody in blood is bound to platelets; consequently direct assays of the patient’s platelets are more sensitive than indirect assays using the patient’s serum and platelets from a healthy control animal.92 Unfortunately direct assays of platelets should be done within 24 hours after blood sample collection. Platelets naturally have some immunoglobulin adsorbed to their surfaces. The amount of platelet-bound immunoglobulin can increase with time after sample collection; consequently false-positive tests can be a significant problem with these assays.156 Positive test results may also occur when immune complexes are adsorbed to platelets. False-negative tests may occur if antibodies have eluted from platelets during processing. Since these assays are generally designed to identify IgG on platelets, a false-negative test may result if IgM rather than IgG antibodies are bound to platelets.132 False-negative tests may occur if assays are done after immunosuppressive therapy is initiated.120 At this time none of the tests for antiplatelet antibodies are as readily available and as cost-effective as the DAT for antierythrocyte antibodies.
Primary Immune-Mediated Disorders
Some degree of immune-mediated cellular destruction occurs in many infectious, parasitic, neoplastic, inflammatory, and drug-induced diseases.104 Disorders presented in this section do not appear to be secondary to other diseases but represent primary immune-mediated disorders.
Transfusion Reactions
DEA 1.1 antibody-antigen interactions result in most of the acute hemolytic transfusion reactions in dogs,1 but transfusion reactions have been reported against DEA 1.2,65 DEA 4,103 and an unclassified common antigen23 on dog erythrocytes. A blood type termed Dal has been reported to be lacking in some Dalmatian dogs but is present in a high percentage of dogs other than Dalmatians. Dalmatians lacking the Dal antigen are likely at risk of delayed, and possibly acute hemolytic transfusion reactions if transfused with Dal antigen-positive blood.150
Incompatibilities in the AB blood group of cats has been recognized to cause transfusion reactions.55,56,76 The A and B alloantigens (blood types) result from the expression of two different alleles at the same gene locus, with A being dominant over B.1 Cats rarely express both type A and type B antigens (type AB) on erythrocytes. The frequency of blood types varies with location and breed of cat. From 0.3% (Northeast) to 4.7% (West Coast) of domestic short- and long-hair cats in the United States are type B, but up to 50% of purebred cats of certain breeds in the United States are type B.1 A blood group antigen termed Mik has been reported in domestic shorthair cats that is capable of inducing a hemolytic transfusion reaction when Mik-positive RBCs are transfused into a Mik-negative recipient cat that has naturally occurring anti-Mik alloantibodies in its plasma.150
Aa and Qa are the most immunogenic alloantigens in horses and presumably the most likely to cause a hemolytic transfusion reaction.133 A-negative pigs exhibit intravascular hemolysis when transfused with A-positive blood.110
For most blood groups in animals, antibody formation occurs only following prior exposure to different erythrocyte alloantigens via transfusion, pregnancy, or vaccination with products containing blood group antigens.71 Consequently adverse transfusion reactions to unmatched erythrocytes generally do not occur at the time of the first blood transfusion. However, the AB and Mik groups in cats and the A group in pigs are characterized by “naturally occurring” antibodies (i.e., antibodies that occur in plasma in the absence of prior exposure to blood from another individual).140 In these cases, hemolytic transfusion reactions can occur at the time of the first blood transfusion. This is especially true in the case of B-positive cats, which have naturally occurring anti-A antibodies of high hemolytic titer. In contrast, cats with type A blood have weak anti-B antibodies in their blood. Type B blood transfusions given to type A cats do not result in severe intravascular hemolysis, but the transfusion is not efficacious because the transfused erythrocytes are phagocytized and removed within a few days.19
Neonatal Isoerythrolysis
Animals with neonatal isoerythrolysis (NI) are healthy at birth but develop hemolytic anemia within a few hours to a few days after they ingest colostrum. Historically, Aa and Qa have been the most common antigens associated with neonatal isoerythrolysis in foals. Mares negative for one of these antigens develop antibodies against them and transfer these antibodies to their foals through colostrum. Hemolysis occurs when the foal inherits the respective antigen from the sire.13 The dams become sensitized to these foreign erythrocyte antigens from leakage of fetal erythrocytes through the placenta during pregnancy or from exposure to fetal erythrocytes of the same blood type during a previous parturition. Generally the first foal born is unaffected, but subsequent foals carrying the same foreign antigen(s) will likely develop hemolytic anemia. Other alloantigens associated with neonatal isoerythrolysis in foals include Db, Dg, Pa, Qb, Qc, and a combination of Qa, Qb, and Qc.14,96 Neonatal isoerythrolysis has been reported in mule foals because of an erythrocyte antigen not found in horses but present in some donkeys and mules.14,143
NI can occur in type A kittens born to primiparous type B queens because all adult type B cats naturally have high anti-A antibody titers. NI appears to be an important cause of neonatal death (“fading kitten syndrome”) in purebred cats from breeds with high frequencies of type B blood (see Table 6-1).18,58 Clinical signs that may be present include hemoglobinuria, pale mucous membranes, icterus, lethargy, weakness, tachypnea, tachycardia, collapse, and death. Tail-tip necrosis may occur in surviving kittens as a result of cold-acting IgM antibodies or localized thrombus formation.15
NI has been recognized in pigs, with antibodies usually directed against alloantigens of the E blood group.140 Naturally occurring neonatal isoerythrolysis has not been reported in cattle, but it occurs in some calves born to cows previously vaccinated for anaplasmosis or other vaccines of bovine origin containing erythrocyte membranes.95
Blood typing of prospective breeding animals can be done to minimize the possibility of NI in offspring. The possibility of offspring developing NI can be evaluated by cross-matching the sire’s erythrocytes with the dam’s serum during pregnancy. If the potential for NI is identified prior to parturition, colostrum can be withheld from the offspring until a cross-match can be done between the erythrocytes of the offspring and the serum of the mother. If an incompatibility is present, the neonatal animal can be foster-fed for 2 days, allowing it to nurse from the mother after antibodies can no longer be absorbed as a result of gut closure.6
Primary Immune-Mediated Hemolytic Anemia
The binding of antibodies and/or complement to erythrocyte surfaces can result in phagocytosis by macrophages and in some case, complement activation and intravascular hemolysis. IMHA may be primary (also called autoimmune hemolytic anemia) or it may occur secondarily to rickettsial, bacterial, protozoal, viral, or hemoplasma infections; neoplasia (especially lymphomas); and toxin or drug exposure.78,98,104 Vaccination with combination vaccines has been incriminated as a trigger of IMHA in dogs,43 but subsequent studies were not able to verify this association.22,24 In an autoimmune response, antibodies are directed against self antigens on erythrocytes. In secondary immune-mediated disorders, the immune response is directed against foreign antigens or altered self antigens, with inadvertent erythrocyte injury.
A diagnosis of IMHA is made if autoagglutination (persisting after saline washing of erythrocytes) is present, a positive DAT test is measured, or flow cytometry for erythrocyte surface immunoglobulin is positive.104 A diagnosis of primary IMHA is reached by ruling out other disorders known to have concomitant IMHA.
About two-thirds of dogs with IMHA appear to have primary IMHA.5,125 In contrast, IMHA in noncanine species is usually a secondary, rather than a primary, disorder.98 Feline leukemia virus (FeLV) and Mycoplasma haemofelis are most commonly associated with IMHA in cats.42,85
Results from multiple studies of many dogs with primary IMHA have been summarized.* Primary IMHA is typically seen in middle-aged dogs (average age 6 years), with intact and neutered female dogs, neutered male dogs, and cocker spaniel dogs being overrepresented. Autoagglutination is reported to occur in about 60% and the DAT is positive in about 70% of dogs with primary IMHA. Spherocytosis is also present in about 75% of dogs with primary IMHA. Although the presence of spherocytosis strongly suggests that an immune-mediated process is present, other causes of spherocytosis—including exposure to venoms, zinc toxicity, transfusion of stored blood, and hereditary disorders—must be ruled out. Spherocytes are accurately recognized only in dogs because the degree of central pallor is naturally less in other domestic animals.
Anemia in IMHA is often severe, with a mean hematocrit value of about 15%. About two-thirds of dogs with primary IMHA have an absolute reticulocytosis. However, a regenerative response to this hemolytic anemia may be lacking if the onset of anemia is acute or if antibodies and/or complement are directed against reticulocytes or bone marrow precursor cells.98 Hyperbilirubinemia is present in about 75% of cases and bilirubinuria is present in nearly all cases. Intravascular hemolysis, as evidenced by hemoglobinemia with hemoglobinuria, generally occurs in less than 20% of cases. In most cases of primary IMHA in dogs, increased IgG antibodies are bound to the erythrocytes, but in some cases IgM and/or complement are also bound to the erythrocytes. IgM antibodies and/or complement are most likely involved if autoagglutination or intravascular hemolysis is present.98
A leukocytosis (mean total leukocyte count about 32 × 103/µL) is present in more than 80% of dogs with primary IMHA. This increase in total leukocyte count is primarily the result of neutrophilia (often with a left shift), but a monocytosis may also be present. Moderate to marked leukocytosis with a left shift indicates probable ischemic necrosis within tissues—including liver, kidney, heart, lung, and spleen—attributable to thromboembolic disease or anemic hypoxia.101
In some instances the concurrent thrombocytopenia also appears to be autoimmune in origin (Evans syndrome).78 Primary IMHA may also be part of SLE, a multisystemic autoimmune disease.53,140
Primary IMHA is much less common in cats than in dogs. Kohn et al.85 have reported findings from 19 cats with primary IMHA. Affected cats were typically young (median age 2 years). The anemia was generally severe (median hematocrit 12%) and often macrocytic (median MCV 56 fL). An absolute reticulocytosis was reported in less than half of the cases. In contrast to dogs, a leukocytosis was present in only 10% of cats, and about 30% of cats exhibited a lymphocytosis. Thrombocytopenia occurred in about 40% of the cats with primary IMHA, and PT and/or APTT times were prolonged in 30% of the cats evaluated. Hyperbilirubinemia occurred in nearly 70% of cats, with hyperglobulinemia reported in about half of the cats with primary IMHA.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree