CHAPTER 60 Immune-Mediated Hemolytic Anemia
ETIOLOGY AND PATHOGENESIS
PRIMARY AND SECONDARY IMMUNE-MEDIATED HEMOLYTIC ANEMIA
In immune-mediated hemolytic anemia (IMHA), the underlying pathogenesis involves destruction of red blood cells (RBCs) by a type II (antibody-dependent cytotoxicity) mechanism. Initiation of IMHA involves presentation of a self-antigen by major histocompatibility complex (MHC) molecules and interactions between autoreactive T- and B-lymphocytes resulting in synthesis of autoantibodies. Immunoglobulin (Ig) G or IgM antibody-coated RBCs may be removed after formation of the membrane attack complex of the terminal pathway of complement (intravascular lysis) or after opsonization by IgG and/or complement C3b and phagocytosis by macrophages in the spleen and liver (extravascular lysis).1
The antibody may attach to the surface of the RBCs by several different mechanisms. Antibodies may be directed against normal erythrocytes or against erythrocytes altered antigenically through interaction with secondary causes. If the stimulus responsible for antibody production can not be identified, the anemia is called primary IMHA (pIMHA) or autoimmune hemolytic anemia (AIHA). The “true” autoantibody binds to a structural component of the erythrocyte membrane (self-antigen) and may develop after primary immunological dysregulation. In dogs, major autoantigenic molecules, such as erythrocyte membrane glycoproteins (glycophorins), and components of the erythrocyte anion exchange channel (band 3) have been identified.2 However, there are no data available in cats to indicate the major autoantigen.
In many cases, there is a specific underlying cause for this antibody binding (secondary IMHA [sIMHA]). Mechanisms of antibody binding in sIMHA are (1) binding to a foreign epitope carried by an infectious agent (e.g., Hemoplasma spp.) or drug that is attached nonspecifically to the cell surface (innocent bystander destruction); (2) a drug (acting as a hapten) binds to a RBC surface molecule, creating a novel epitope recognized as foreign by the immune system (modified-self); (3) binding of a drug or microbe causes exposure of a previously cryptic determinant; and (4) alloantibody binding to a blood group antigen. Additionally, Ig or immune complexes may be absorbed nonspecifically onto the surface of the erythrocyte but will not necessarily mediate cell destruction (Figure 60-1).1
sIMHA in cats can be triggered by infectious agents, such as feline leukemia virus (FeLV), coronaviruses that induce feline infectious peritonitis (FIP), feline immunodeficiency virus (FIV), Hemoplasma spp., and Hepatozoon infection; inflammatory diseases (e.g., sterile and nonsterile abscesses, cholangiohepatitis, pyothorax, polyarthritis, nephritis, or lymphocytic enteritis), drugs (e.g., propylthiouracil, methimazole); or tumors (e.g., malignant lymphoma, erythroid leukemia, mast cell leukemia, or lymphoid leukemia). IMHA also has been described in cats suffering from amyloidosis and myelodysplasia.3–12 Therefore an extensive search for an underlying disorder or trigger is always warranted, and IMHA only should be defined as autoimmune in the absence of identifiable underlying factors.
Recent vaccination (within 1 month) has been implicated as a trigger for immune-mediated diseases such as IMHA13 and polyarthritis14 in dogs. Vaccination against calicivirus has resulted in polyarthritis in cats.15 Therefore it appears likely that vaccines enhance a smoldering immune process. Three of 19 cats with pIMHA had been vaccinated in the 4 weeks before initial presentation.11 However, it is difficult to define a vaccine reaction, and the mechanism underlying this association has not been ascertained.
IMHA and immune-mediated thrombocytopenia (IMTP) occur most commonly in isolation, but the two conditions may occur together (Evans’ syndrome), which has been described in a small number of cats.16,17 Moreover, IMHA can be part of the multisystemic autoimmune disease (e.g., systemic lupus erythematosus).18,19
IMHA often is associated with a strongly regenerative bone marrow erythroid response; however, a high percentage of cats do not have reticulocytosis at the time of diagnosis.11 One possible reason for this may be peracute IMHA because 3 to 4 days are required for a substantial reticulocyte response to occur after the onset of anemia.20 An absence of reticulocytosis in animals with anemia of greater than 5 days duration has been attributed to immune reactions directed against erythroid precursor cells. This can be due either to nonregenerative IMHA (associated with bone marrow erythroid hyperplasia or erythroid maturation arrest) or to pure red cell aplasia (PRCA) that is characterized by erythroid aplasia in the bone marrow.21,22
PRCA is an uncommon hematological syndrome characterized by severe anemia, reticulocytopenia, and leukocyte and platelet counts that are within reference ranges. PRCA can be primary (idiopathic) or secondary in origin, and secondary PRCA has been reported in cats infected with FeLV subtype C. Three of six cats in one study of PRCA were Coombs’ test–positive, supporting the theory of immune-mediated pathology in idiopathic cases of PRCA.21
Alloimmune hemolytic anemias, such as hemolytic transfusion reactions and neonatal isoerythrolysis, are caused by specific anti-erythrocytic alloantibodies. Unlike dogs, cats possess naturally occurring antibodies (alloantibodies) against the blood group they are lacking (see Chapter 61). These alloantibodies can lead to incompatibility reactions, such as hemolytic transfusion reactions, when incompatible transfusions are administered. Cats with blood type B who receive type A blood may develop a severe acute hemolytic transfusion reaction, with clinical signs such as lethargy, bradycardia, dyspnea, cardiac arrhythmia, salivation, vomition, defecation and urination, and neurological disorders. Death may occur during this phase. If the cats survive, they develop tachycardia, tachypnea, hemoglobinemia, and hemoglobinuria. If type A cats receive blood from type B cats, only mild incompatibility reactions, such as restlessness, tachycardia, and tachypnea, may be observed. However, the transfusion is not efficient because of the rapid destruction of erythrocytes.23,24 These alloantibodies are the cause of neonatal isoerythrolysis, a very specific form of IMHA, Neonatal isoerythrolysis is an important disorder for breeders because type A kittens born to a type B queen are at risk. Besides the well-characterized AB blood grouping system, a newly recognized blood group, the Mik red cell antigen, with a corresponding clinically relevant, naturally occurring alloantibody, has been described in association with hemolysis.25
CATEGORIES OF IMMUNE-MEDIATED HEMOLYTIC ANEMIA
Classically, IMHA has been subdivided into five main categories based on the thermal reactivity of the anti-RBC antibodies and their major clinical effects at optimal temperature. This categorization system is derived by extrapolation from human beings; however, the five categories have been described in small animals. In cats, as well as in other species, IMHA typically is caused by warm reactive antibodies that exert their effects at body temperature. Three different categories have been described (Class I to III). Cold reactive antibodies are only reactive at cold temperatures; two categories (Class IV to V) have been described (Table 60-1).26
Table 60-1 Categories of Immune-Mediated Hemolytic Anemia
| Category | Antibodies Involved | Pathomechanism |
|---|---|---|
| Class I | IgG, IgM; warm reactive | Autoantibodies agglutinate RBCs at body temperature; agglutination may be seen when a drop of blood is placed on a glass slide; RBCs are destroyed mainly by phagocytosis in the spleen (extravascular hemolysis). |
| Class II | IgM; warm reactive | Antibodies activate complement and destroy RBCs by intravascular hemolysis causing hemoglobinemia and hemoglobinuria. |
| Class III | IgG; warm reactive | Antibodies bind to RBCs at 37° C; they do not activate complement or agglutinate RBCs (affected cells are removed by splenic macrophages, extravascular hemolysis). |
| Class IV | IgM; cold reactive | Antibodies agglutinate RBCs at cold temperatures (“cold-agglutinins”); agglutination can occur within the vasculature of the extremities. |
| Class V | IgM, cold reactive | IgM antibodies will bind RBCs when chilled to 4° C but will not agglutinate them; these antibodies can activate complement leading to intravascular hemolysis. |
IgG, Immunoglobulin G; IgM, immunoglobulin M; RBCs, red blood cells.
Data from Tizard IR: Veterinary immunology, Philadelphia, 2004, Saunders.
EPIDEMIOLOGY
IMHA was reported to be uncommon in cats. However, very few data are available regarding the occurrence of the different types of anemia in cats. One hundred cats who presented to the Small Animal Clinic, Freie Universität Berlin over a 2-year period with a hematocrit (Hct) <25 per cent were studied prospectively. Twenty-four cats suffered from hemolytic anemia: 12 of them from pIMHA, three from sIMHA, three cats had FIP, and one cat each with lymphoma, severe hypophosphatemia, and Heinz body anemia. The cause of hemolytic anemia remained unknown in three cats. The following diagnoses were obtained in the other cats: anemia of inflammatory disease (29 per cent), acute blood loss anemia (22 per cent), chronic blood loss anemia (2 per cent), chronic kidney disease (9 per cent), nonregenerative anemia caused by retrovirus infection/bone marrow disease (9 per cent), and cause of anemia unknown (5 per cent).9 In a retrospective study performed at the Utrecht University, all Coombs’-positive cats presented over an 11-year period were evaluated. Sixty-seven cats with underlying diseases were diagnosed with sIMHA and 29 cats were diagnosed with idiopathic IMHA. These two studies and other reports suggest that pIMHA and sIMHA occur more frequently in cats than recognized previously.10,27
SIGNALMENT
The age of 19 cats with pIMHA ranged from 6 months to 9 years (mean 3.2 years); 12 cats were younger than 3 years.11 In other studies (involving five and 25 cats), the average age was 3 and 6.2 years, respectively.27,28 Domestic Shorthair cats were affected primarily. A slightly higher incidence of pIMHA was reported in male cats.3,11,27 The age of nine cats with idiopathic PRCA ranged from 8 months to 3 years.21
HISTORY
Most owners of the 19 cats with pIMHA sought veterinary care because of nonspecific clinical signs such as lethargy (n = 19) and inappetence (n = 13). Other clinical signs were pica (n = 5) and rarely vomiting (n = 2), pruritus, dyspnea, epistaxis, polydipsia, and obstipation (one cat each). Before initial evaluation, the cats had been sick between 1 and 56 days (median 8.5 days). Three of 19 cats with pIMHA had been vaccinated 3 to 4 weeks before initial presentation.11 However, the association between vaccination and disease might have been coincidental.
CLINICAL AND LABORATORY FINDINGS
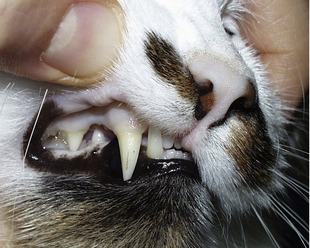
Cats often are presented in a late stage of the disease because the major clinical signs of lethargy and inappetence are nonspecific. Moreover, cats appear to tolerate low Hct values better than dogs. Further findings often include pale mucous membranes (Figure 60-2), mild-to-moderate systolic heart murmurs (degree II-IV/VI), and rarely, hypothermia or hyperthermia. Heart murmurs may be due to changes in rheological properties of the blood because of severe anemia; however, it is possible that chronic anemia may lead to a secondary cardiomyopathy. Compared to dogs, icterus is rare in affected cats and was detected in only two of 19 patients, suggesting a slow, well-compensated hemolytic process in most cases.11
Cats reported in the literature suffered from extravascular hemolysis; intravascular hemolysis occurs less frequently in cats than in dogs, and a careful search for sIMHA (e.g., a result of hemoplasmosis) is warranted in these cases (Figure 60-3).
A mean Hct of 12 per cent was reported in 30 cats with pIMHA at initial presentation.27,28 In another study with 19 cats, the Hct ranged from 6 to 22 per cent (median 12 per cent); 79 per cent of the cats had a severe anemia with a Hct less than 15 per cent.11 Twenty-nine cats with pIMHA had Hct values ranging from 6 to 23 per cent (median 9.5 per cent), which was significantly lower than the Hct values of a group of cats with sIMHA (n = 67; range 4 to 38 per cent, median 14 per cent).10
The median Hct of nine cats with idiopathic PRCA was 7 per cent (range 6 to 15 per cent).21 In a study by Weiss (2008), the mean Hct of 29 cats with PRCA was 7 per cent (SD ± 2); twenty-four cats were diagnosed with nonregenerative IMHA with erythroid hyperplasia and four cats had nonregenerative IMHA with erythroid maturation arrest. The mean Hct in these cats was 13 and 14 per cent, respectively.22
Regeneration of RBCs in patients with hemolytic anemia is characterized by macrocytic and either hypochromic or normochromic erythrocytes. At initial evaluation, 13 of 19 cats with pIMHA had an increased mean corpuscular volume (MCV), but only eight of these patients had increased numbers of aggregated reticulocytes.11 However, MCV also can be increased in patients with severe agglutination because it is difficult for cell counters to identify single RBCs resulting from agglutination.
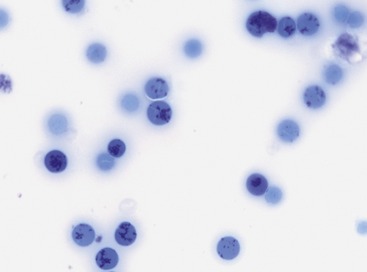
Median absolute aggregate and punctate reticulocyte counts at initial presentation in two studies with 19 and 25 cats with pIMHA were 16,000 and 72,600/µl and 46,000 and 248,070/µl, respectively (Figures 60-4 and 60-5). In 11 of 19 cats (58 per cent) with pIMHA, the anemia was nonregenerative at initial evaluation, which makes a diagnosis of hemolytic anemia more challenging. The aggregated reticulocyte counts of these 11 cats ranged from 0 to 28,600/µl and increased in 10 cats after 7 to 33 days to greater than 40,000/µl.11 Because bone marrow results of these cats were not available, some patients might have suffered from nonregenerative IMHA or PRCA.22
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree