CHAPTER 9 Immune-Mediated Disorders
Immune-mediated dermatoses are well-recognized but uncommon skin diseases in the horse.* These dermatoses have been reported to account for about 4% of all equine dermatoses examined by the dermatology service at a university practice.8,9 They have been subdivided into primary or autoimmune, and secondary or immune-mediated disorders, the latter believed to be primarily diseases wherein tissue destruction results from an immunologic event that is not directed against self-antigens.
In autoimmune disease, antibodies or activated lymphocytes develop against normal body constituents and will induce the lesions of the disease by passive transfer. The precise cause(s) of autoimmune disease is(are) not known. These diseases appear to develop spontaneously, and precipitating causes are rarely obvious. Various theories concerning the cause of autoimmune diseases include: hidden antigens in cells and tissues, antigens generated by molecular changes, molecular mimicry, receptor editing, alterations in antigen processing, failure of regulatory control, virus-induced abnormal reactivity, and microchimers.13 The mechanisms of tissue damage include types I-IV hypersensitivity reactions.13
Diagnosis of immune-mediated skin disease
The diagnosis of these dermatoses requires demonstration of characteristic dermatopathologic changes and, optimally, the autoantibodies, immune complexes, or mediators (e.g., cytotoxic T-cells) involved in the immunologic injury. Establishing the presence of characteristic dermatopathology requires skin biopsy (see Chapter 2). In general, the following guidelines should be observed12:
Establishing the mediator of the immunologic damage may require biopsy and/or analysis of the patient’s serum for auto- or abnormal antibodies. The biopsies for immunopathologic examination often must be processed in special ways that may require fresh tissue, frozen tissue, or specially fixed samples, depending on the test that will be performed. The veterinary immunopathology laboratory will be able to tell you what is required. Tests used to detect the presence of autoantibodies or various immunoreactants (e.g., immunoglobulins [Igs], complement components, microbial antigens) in skin lesions include immunofluorescence and immunohistochemical (e.g., immunoperoxidase) methods.12
Biopsy specimens for direct antibody testing should be selected from areas not secondarily infected and generally representing the earliest lesion typical for that disease; a possible exception is discoid lupus erythematosus (DLE), wherein older lesions may be preferred. Vasculitis lesions under 24-h old are best. For pustular or bullous diseases, the primary lesion and the adjacent normal skin or erythematous skin are sampled. Samples for direct immunofluorescence testing need to be fixed and mailed in Michel fixative. Samples for direct immunoperoxidase testing may be formalin-fixed. The results of studies of tissues processed by quick-freezing and of those kept in Michel fixative for up to 2 weeks are comparable.12 Studies in horses suggest that specimens may be reliably preserved in Michel fixative for at least 7-14 days, and in some instances, specimens have been successfully preserved for 4-8 years.12 The pH of Michel fixative must be carefully maintained at 7.0-7.2 to ensure accurate results. Samples for direct antibody detection should be sent to a veterinary immunopathology laboratory.
Testing for abnormal antibody or immune complex deposition is considered highly valuable in human medicine and for many of the immune-mediated dermatoses. For the similar equine diseases, however, their value has been considerably less. These tests are fraught with numerous procedural and interpretational pitfalls, including method of specimen handling, choice of substrates used, method of substrate handling, specificity of conjugates, fluorescein-protein-antibody concentrations, and unitage of conjugates. The incidence of positive results in the equine disorders typically varies from about 25% to 90% for direct immunofluorescence testing.12 Positive results are much more commonly achieved with the immunoperoxidase method. However, with this technique the incidence of false-positive results is also much higher. In fact, the intercellular and basement membrane zone deposition of Igs or complement components can be detected from time to time in a wide variety of inflammatory dermatoses.12 These tests do not need to be routinely done in the work-up of a suspected case of immune-mediated skin disease in a horse.12 Results of immunopathologic testing can never be appropriately interpreted in the absence of histopathologic findings. On the other hand, histopathologic findings are sufficiently characteristic to be diagnostic in the majority of cases. The clinician’s time and the owner’s money are better spent in the careful selection and procuring of representative skin specimens and their forwarding to a knowledgeable dermatopathologist.
Autoimmune dermatoses are classified based on the specific autoallergen being targeted.1,2,12,13 This cannot be determined by routine immunofluorescence or immunohistochemical testing. Specific identification requires techniques such as immunoprecipitation, immunoblotting, and antigen-specific enzyme-linked immunosorbent assay (ELISA). This is rarely done in routine veterinary diagnostic laboratories and is limited to certain veterinary immunologic research laboratories. As a result, different diseases or variants that share clinical, histopathologic, and routine immunopathologic features may still be lumped together.
Indirect immunofluorescence testing (testing serum for the presence of circulating autoantibody) has been positive in less than 50% of affected horses and is not recommended as a cost-effective test.12 In addition, these tests can be positive in normal horses and horses with dermatoses of nonimmune-mediated origin.12
Therapy of immune-mediated skin diseases
The drugs used to treat immune-mediated skin diseases are generally referred to as immunosuppressive agents. However, for some of these drugs, their exact mechanism is unknown. They may act in methods different from those of the more classic immune-suppressive agents. They are considered together because, whatever their mechanism of action, they share the features of being beneficial in managing the immune-mediated skin diseases. Glucocorticoids are the most common class of drugs used as immunosuppressive agents. They are discussed in Chapter 3. The following immunomodulating drugs are used in the horse, but very little specific data are available.
Azathioprine
Azathioprine (Imuran, Glaxo Wellcome) is a synthetic modification of 6-mercaptopurine that is given orally (PO).2,12,16 It is metabolized in the liver to 6-mercaptopurine and other active metabolites; 6-mercaptopurine is then metabolized by three enzyme systems. Xanthine oxidase and thiopurine methyltransferase (TPMT) produce inactive metabolites. Humans and possibly dogs that have absent (homozygous) or low (heterozygous) TPMT activity are more likely to develop myelosuppression. The drug antagonizes purine metabolism, thereby interfering with DNA and RNA synthesis. Azathioprine primarily affects rapidly proliferating cells, with its greatest effects on cell-mediated immunity and T-lymphocyte-dependent antibody synthesis. Primary antibody synthesis is affected more than secondary antibody synthesis.
Azathioprine is a potent drug with potential toxicities in humans and companion animals, which include anemia, leukopenia, thrombocytopenia, vomiting, hypersensitivity reactions, hepatotoxicity, pancreatitis, skin rashes, and alopecia. Azathioprine was administered PO, daily for 30 days and then every other day for an additional 30 days, to six healthy horses.16 Bioavailability was low, and no clinical, hematologic, or biochemical abnormalities were observed. Adverse reactions to azathioprine when used in horses with immune-mediated dermatoses have not been reported.*
In horses, azathioprine may be beneficial for pemphigus foliaceus, pemphigus vulgaris, bullous pemphigoid, both types of lupus erythematosus, and other autoimmune and immune-mediated disorders, such as vasculitis.* It is most commonly used in cases of pemphigus foliaceus and vasculitis that do not respond to glucocorticoids. Azathioprine is usually not used alone, but is combined with systemic glucocorticoids. There may be a lag phase, with clinical improvement occurring in 3-6 weeks. After remission is achieved, the dosages of both drugs are tapered, but initially, unless side effects are a problem, the glucocorticoid dosage is tapered to levels approaching 1 mg/kg every 48 h (prednisolone equivalents). The oral dosage of azathioprine for horses is 2-3 mg/kg every 24 h until clinical response is achieved, and then it is continued every other day for a month or longer. Slow tapering to the lowest dose possibly decreases the risk of side effects and the expense of therapy. Glucocorticoids can be given on the alternate days when azathioprine is not given.
Chrysotherapy
Chrysotherapy is the use of gold as a therapeutic agent.2,12 Gold compounds are capable of modulating many phases of immune and inflammatory responses, but the exact mechanisms of this effect are unknown. In humans and dogs, the parenteral form is 100% absorbed, but has only a 6-day half-life in blood. It is 95% protein-bound and is well-distributed to cells of the mononuclear phagocytic system, liver, spleen, bone marrow, kidneys, and adrenal glands. Much lower levels are detected in skin. In humans, serum concentrations reach a plateau after 1-2 months of weekly aurothioglucose injections, and urinary excretion can be detected for as long as 1 year after chronic therapy is stopped.
Toxic effects are worrisome in humans, because 33% of patients have some adverse reaction, although 80% of these are minor.2 Most common are skin eruptions, oral reactions, proteinuria, and bone marrow depression. During the induction phase, a hemogram and urinalysis should be checked weekly and monthly thereafter. Side effects have not been reported in horses.* In laboratory rodents, gold is known to cross the placenta, be secreted in milk, and to be teratogenic.
Aurothioglucose and gold sodium thiomalate have been reported to be effective for the treatment of cases of equine pemphigus foliaceus, including those unresponsive to glucocorticoids.† Horses are given 1 mg/kg intramuscularly (IM) weekly until remission occurs. If no response is seen after 16 weeks of therapy, the aurothioglucose is unlikely to be of benefit. After remission, one dose is given every 2 weeks for a month and then once monthly for several months. It is advisable to halt medication administration eventually for observation, because some patients go into complete remission,12,24,30 whereas other animals can be maintained on a reduced dosage. Two points of caution: (1) the treatment takes up to 16 weeks for beneficial effects to occur, so other medication, typically glucocorticoids, should be maintained, if needed, at full dosage until this lag period is passed; and (2) gold compounds should not be administered simultaneously with other cytotoxic drugs (such as azathioprine), because toxicity is thereby enhanced.
Pentoxifylline
Pentoxifylline (Trental, Hoechst-Roussel or generic) is a methylxanthine derivative that produces a variety of physiologic changes at the cellular level.2-4,12 Immunomodulatory and rheologic effects include increased leukocyte deformability and chemotaxis; decreased platelet aggregation; decreased leukocyte responsiveness to IL-1 and tumor necrosis factor (TNF)-α; decreased production of TNF-α from macrophages; decreased production of IL-1, IL-4, and IL-12; inhibition of T- and B-lymphocyte activation; and decreased natural killer (NK) cell activity. It also has been shown to inhibit T-cell adherence to keratinocytes. In humans and dogs, these effects are beneficial in the treatment of peripheral vascular disease, vasculitis, atopic dermatitis, and allergic contact dermatitis. Pentoxifylline also has been used for a variety of inflammatory diseases such as necrobiosis lipoidica, granuloma annulare, and brown recluse spider bites. The drug also affects wound healing and connective tissue disorders through increased production of collagenase and decreased production of collagen, fibronectin, and glycosaminoglycans. In humans, the drug has been used to treat scleroderma and keloids.
Pentoxifylline has been used in the horse for the treatment of atopic dermatitis, vasculitis, erythema multiforme, pemphigus foliaceus, and sarcoidosis.12,15,25 Most reports are anecdotal. Pentoxifylline improved the respiratory function of horses with chronic obstructive pulmonary disease (“heaves”) maintained in an unfavorable environment.3 Pentoxifylline is available in a 400 mg coated tablet. It is usually crushed and dosed at 10 mg/kg every 12 h.3,4,12 It should be administered for a minimum of 4 weeks. Side effects are minimal and may include transient sweating, behavioral change, and conjunctivitis.
Autoimmune diseases
Pemphigus Complex
The pemphigus complex is a group of uncommon to very rare autoimmune diseases described in horses that is comparable to the human, canine, and feline diseases.1,2,12 These disorders are vesiculobullous to pustular disorders of the skin or mucous membranes characterized by acantholysis (loss of cohesion between keratinocytes).
Cause and pathogenesis
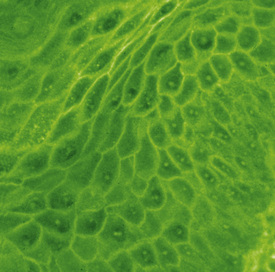
The pemphigus complex is characterized histologically by intraepithelial acantholysis leading to pustule or vesicle formation, and immunologically by the presence of autoantibodies (both bound in the skin and circulating in the serum) that target various molecules that are crucial to the integrity of keratinocyte cell-to-cell adhesion.1,2 The clinical lesions, both in severity and in body location, appear to relate to which components of the desmosome the autoantibodies are targeting. For instance, humans with mucosal-dominant pemphigus vulgaris have antibodies against desmoglein III, those with pemphigus foliaceus produce antibodies against desmoglein I, and patients with mucocutaneous pemphigus vulgaris produce both.1,2 As pemphigus vulgaris progresses to cutaneous involvement, antidesmoglein I antibodies are produced, possibly due to epitope spreading.
Pemphigus antigens are heterogeneous (85-260 kDa), present in all mammalian and avian skin, and those identified specifically are associated with desmosomal components.1,2 In humans, regional variation exists in the expression of both pemphigus foliaceus and pemphigus vulgaris antigens, which also differ from each other. This regional difference and the specific profile of the patient’s autoantibodies correlates with, and helps to explain, the distribution of lesions seen in clinical disease. The pemphigus vulgaris and pemphigus foliaceus antibodies from human patients reproduce their respective clinical, histopathologic, and immunopathologic syndromes when injected into neonatal mice. Antibodies to some of these antigens are not, however, associated with pathology.
The pathomechanism of blister formation in pemphigus is not entirely clear.1,2 Autoantibodies against desmogleins and/or other adhesion-related molecules (e.g., desmocollins, desmoplakin, envoplakin, periplakin, plakoglobin, pemphaxin, cholinergic receptors) cause lesions by interfering with cell-to-cell adhesion functions of these molecules or with their role in desmosome assembly.1,2,19
What initiates the autoantibody formation is still unknown, though a virus spread by an insect vector is suspected in an endemic form of pemphigus foliaceus (fogo selvagem) seen in South America.1,2 It has been suggested that the black fly may play the role of vector. This hypothesis has been supported by an epidemiologic study correlating exposure to black flies as a risk factor for the development of endemic pemphigus foliaceus. In some instances, equine pemphigus foliaceus has been known to occur, intensify, or recur on a seasonal (warm weather) basis,* or occur in a group of horses that had Culicoides hypersensitivity for 1-3 years.12 Such observations suggest that insects (Culicoides gnats, Simulidae spp., etc.), seasonal antigens such as pollens and molds (atopic dermatitis), or ultraviolet (UV) light may also play a role in some cases of equine pemphigus.
Genetic factors in humans and dogs also appear to be important.1,2 Breed predilections have not been established in horses. Other factors thought to be involved in the pathogenesis of some cases of pemphigus are drug provocation (to include vaccines, dewormers, and supplements), UV light, and stress.1,2,10,29 One horse developed pemphigus foliaceus after a third course of penicillin and spontaneously resolved after penicillin administration was stopped.17
Diet has been implicated as a cause of pemphigus in humans.26 The molecular structure of many food ingredients is similar to that of known pemphigus-inducing drugs. For instance: thiols contained in garlic, onion, leek, and chive; isothiocyanates in mustard, horseradish, turnip, radish, cabbage, cauliflower, and Brussel sprouts; phenols in mango, cashews, and many food additives; tannins in tea, coffee, ginseng, certain berries, banana, pear, apple, and avocado. Interestingly, most humans with fogo selvagem live in close proximity to rivers, many of which contain high levels of tannins due to decomposing leaves and other vegetable matter.26 Heat and humidity cause tannin decomposition. Perhaps consumption of such substances is more important than black flies.
Diagnosis
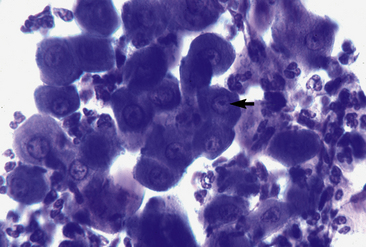
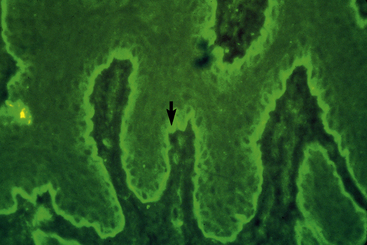
The pemphigus complex is uncommon to rare in horses, accounting for about 2% of all equine skin disorders seen at the CUHA.8,9 In general, the various forms of pemphigus have relatively distinct clinical differences. Certain diagnostic features, however, can be applied to the whole group. The most important diagnostic aspects are the history, physical examination, and histopathologic findings. Detection of pemphigus antibody by direct immunofluorescence or immunohistochemical testing may also be helpful, but owing to costs, technical problems, and relatively poor diagnostic sensitivity and specificity, those tests are not routinely recommended. If they are performed, however, all the pemphigus variants should show an intercellular deposition of IgG or complement components (Fig. 9-1).12 Occasionally, Igs of other classes may be found. Indirect immunofluorescence testing is frequently negative. Microscopic examination of direct smears from intact vesicles or pustules or from recent erosions often reveals numerous nondegenerate neutrophils and/or eosinophils and numerous acantholytic keratinocytes.12 One or two acantholytic keratinocytes may be seen in an occasional high-power microscopic field during the microscopic examination of any suppurative condition, but when these cells are present in clusters (“rafts”) or large numbers in several microscopic fields, they are strongly suggestive of pemphigus. Marked acantholysis has rarely been observed cytologically in cases of equine dermatophytosis.23
Skin biopsy findings may be diagnostic or strongly supportive in pemphigus.12 Intact vesicles, bullae, or pustules are preferred. Because these fluid-filled lesions are so fragile and transient, it may be necessary to hospitalize the animal so that it can be scrutinized every 2-4 h for the presence of primary lesions. In some cases, the diagnosis may be made by selecting recently exudative, crusted areas of skin, wherein the acantholytic keratinocytes are found within the crusts and exudate. Multiple biopsy specimens and serial sections will greatly increase the chances of demonstrating diagnostic histopathologic changes.
Electron microscopic examination of pemphigus lesions suggests that dissolution of the intercellular cement substance is the initial pathologic change, followed by the retraction of tonofilaments, disappearance of desmosomes, and acantholysis.2,12
Results of routine laboratory determinations (hemogram, serum chemistries, urinalysis, serum protein electrophoresis) are nondiagnostic, often revealing mild-to-moderate leukocytosis and neutrophilia, mild nonregenerative anemia, mild-to-moderate hypoalbuminemia, mild-to-moderate hyperglobulinemia and hyperfibrinogenemia, and mild-to-moderate elevations of α2, β, and γ globulins.12,27
Clinical management
Therapy of equine pemphigus is often difficult, requiring large doses of systemic glucocorticoids with or without other potent immunomodulating drugs.* Close physical and hematologic monitoring of the patient is critical. Therapy often must be maintained for prolonged periods, if not for life. Thus, the therapeutic regimen must be individualized for each patient, and owner education is essential.
Large doses of glucocorticoids (2-4 mg/kg prednisolone or prednisone, or 0.2-0.4 mg/kg dexamethasone PO every 24 h in the morning) will induce remission in most horses. Marked clinical improvement should be seen within 10-14 days. When glucocorticoids are ineffective or undesirable, other immunomodulating drugs can be used in an attempt to reduce the dosage or eliminate the need for the former. Chrysotherapy (gold salts) or azathioprine are most commonly used (see Therapy of Immune-Mediated Skin Diseases).† Pentoxifylline and omega-3/omega-6 fatty acid-containing products may also be useful.12,29
Exposure to UV light can exacerbate or even precipitate pemphigus.2,12 Thus, photoprotection is an important therapeutic adjunct. Avoidance of sunlight between 8 AM and 5 PM is helpful.
Pemphigus foliaceus
Pemphigus foliaceus is the most common form of pemphigus and the most common autoimmune dermatosis in the horse.‡ It is, however, an uncommon disease, accounting for only 1.9% of the equine skin diseases seen at the CUHA. In humans and a minority of dogs, the major pemphigus foliaceus antigen is desmoglein 1, a 150-kDa glycoprotein from the cadherin group of adhesion molecules.1,2 The exact antigen in the horse has not been identified. Desmoglein 1 has been identified in the muzzle skin of healthy horses.21 There are no apparent breed, sex, or age predilections, and the disease has been reported in horse 2-months to 25-years old.
Some cases of equine pemphigus foliaceus occur, intensify, or recur on a seasonal (warm weather) basis,12,25,27,29 suggesting a triggering role for UV light, insects, and/or pollens and molds. Anecdotal reports indicate that, in many cases, a triggering event (drug administration [to include vaccines, dewormers, supplements], variety of systemic diseases, “stressful situations”) precedes the onset of disease.14,29 Anecdotal reports7 also indicate that foals of mares with pemphigus foliaceus have acquired a transient, self-limited form of the disease because of colostral transfer of autoantibodies. These foals may actually have had the syndrome of neonatal ulcerative dermatitis, thrombocytopenia, and neutropenia (see Chapter 14). Rarely, dermatophytosis due to T. equinum can produce clinical and pathologic syndromes (acantholytic dermatophytosis) similar to pemphigus foliaceus (see Chapter 5).15,23,29
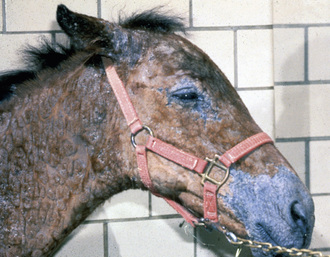
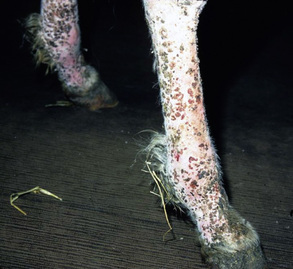
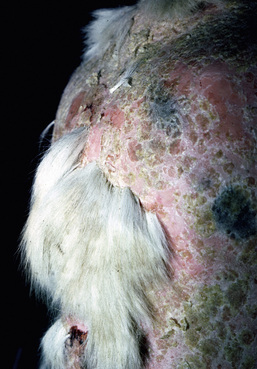
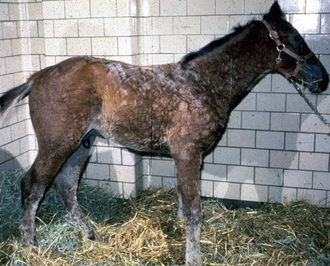
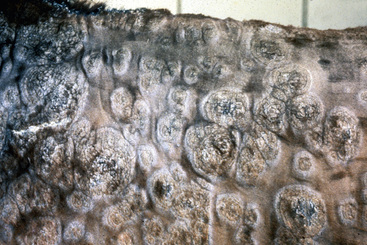
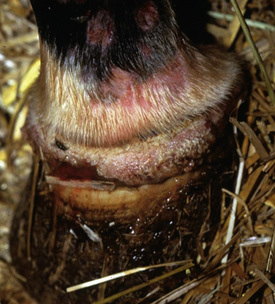
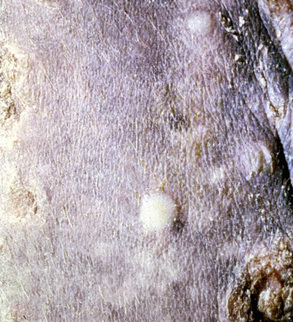
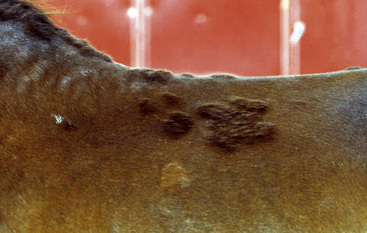
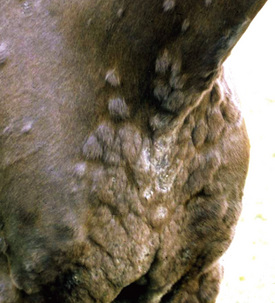
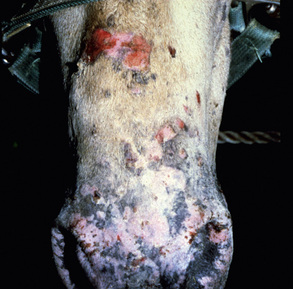
Skin lesions commonly begin on the face (Fig. 9-2), legs, and/or ventrum (Fig. 9-3), and frequently become generalized within 1-3 months (Figs. 9-4–9-7). In some cases, lesions are localized to the face or coronary bands (Fig. 9-8) for long periods.* Preputial and mammary areas may be targeted in some cases. The primary skin lesions are vesicles, bullae, or pustules (Figs. 9-9 and 9-10). However, due to the fragile and transient nature of these lesions, the clinician typically sees annular thick crusts, annular erosions with or without epidermal collarettes, annular areas of alopecia, and variable degrees of oozing, matted hair coat, and scaling. In some horses, early lesions are tufted papules and crusts over the withers, saddle area (Fig. 9-11), or brisket (Fig. 9-12). Some cases present with extensive exfoliative dermatitis without distinct annular primary or secondary lesions (Fig. 9-13). It has been reported that transient, persistent, or recurrent urticaria can precede the development of typical pemphigus lesions for days to weeks.12,15,29
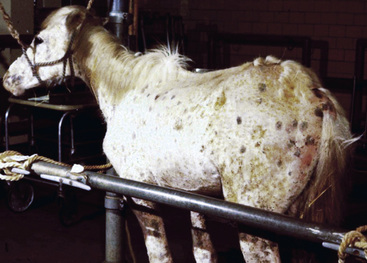
Figure 9-4 Same horse as in Fig. 9-3. Generalized exfoliative dermatitis and relative sparing of the mane and tail.
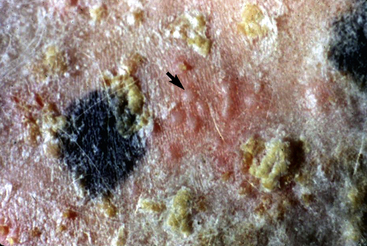
Figure 9-9 Same horse as in Figs. 9-3 to 9-5. Intact vesicles (arrow) on alopecic, erythematous, crusted thoracic skin.
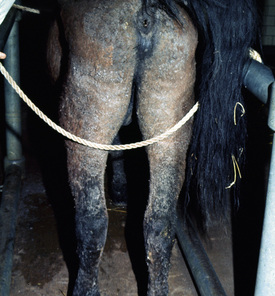
Up to 50% of the cases have varying degrees of edema of the distal limbs (see Fig. 9-8) and ventral abdomen.6,12,15,29 The degree of edema may be out of proportion to, or occur in the absence of, surface lesions. Swellings may be painful and associated with lameness.6 Pruritus and pain may or may not be present. Skin lesions may be exacerbated in warm, humid, sunny weather. Some animals experience spontaneous waxing and waning of their skin disease.12,15,30 Up to 50% of the horses manifest variable systemic signs, including depression, lethargy, poor appetite, weight loss, and fever.* One horse with pemphigus foliaceus was found to have erosions in the esophagus and esophageal zone of the stomach.12
Diagnosis
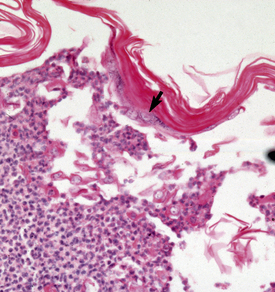
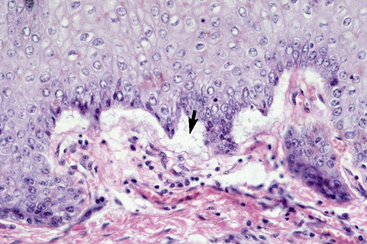
The definitive diagnosis is based on history, physical examination, direct smears (Fig. 9-14), skin biopsy, immunofluorescence or immunohistochemical testing, and demonstration of the antigen being targeted (desmoglein 1 in humans). Pemphigus foliaceus is characterized by intragranular or subcorneal acantholysis with resultant cleft and vesicle or pustule formation (Figs. 9-15 and 9-16).12,14,27,30 Within the vesicle or pustule, cells from the stratum granulosum may be seen attached to the overlying stratum corneum (granular cell “cling-ons”) (Fig. 9-17). Neutrophils are usually the predominant inflammatory cell. A minority of cases will have tissue eosinophilia.12,20,27 Other helpful histopathologic findings that may be seen include: (1) neutrophilic and/or eosinophilic exocytosis and microabscess formation within the epidermis or follicular outer root sheath or both; (2) frequent involvement of the follicular outer root sheath in the acantholytic and pustular process (pustular mural folliculitis); (3) acantholytic, dyskeratotic granular epidermal cells (“grains”) at the surface of erosions; and (4) surface crusts containing numerous acantholytic keratinocytes singly or in clusters (Fig. 9-18). It must be emphasized that the rare cases of acantholytic dermatophytosis can be histologically indistinguishable from pemphigus foliaceus (see Chapter 5). Hence, sections must be scrutinized for the presence of fungal elements, and special stains and fungal cultures may be indicated.
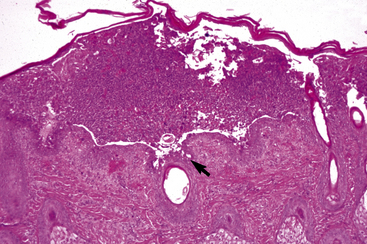
Figure 9-15 Pemphigus foliaceus. Skin biopsy reveals large subcorneal pustule that also involves hair follicles (arrow).
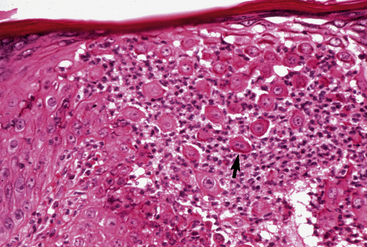
Figure 9-16 Same biopsy specimen as in Fig. 9-15. Numerous acantholytic keratinocytes (arrow) and neutrophils in the pustule.
In many cases of equine pemphigus foliaceus, the remarkable ventral or limb edema is not explained by hypoalbuminemia.12,27 Skin biopsies taken from some of these animals has revealed a vasculitis/vasculopathy.12,27
Clinical management
Rarely, equine pemphigus foliaceus will resolve spontaneously.12,14,20,25 Anecdotal reports indicate that this may occur more commonly in foals.6 The rare horse will wax and wane spontaneously.12,30 However, the vast majority of horses require aggressive treatment. The age at onset may be of prognostic significance.* In general, younger horses (1-year old or less) may have less severe disease, may respond better to treatment, and may eventually remain in remission when treatment is withdrawn.
In most cases, the initial treatment of choice is oral prednisolone or prednisone (2-4 mg/kg PO every 24 h [see Chapter 3]).† The induction dose should be maintained until the disease is inactive (usually 10-14 days). Following induction, the dosage is tapered to an alternate-morning regimen (see Chapter 3). Patients failing to respond to prednisolone or prednisone may respond to dexamethasone (0.2-0.4 mg/kg PO every 24 h). Dexamethasone is not as safe as prednisolone or prednisone for chronic alternate-morning maintenance therapy (see Chapter 3). Hence, if dexamethasone was used to achieve remission, one should attempt to substitute prednisolone or prednisone for maintenance. In horses with hypoalbuminemia, it may be preferable to begin glucocorticoid therapy with prednisolone.15,27
In horses that do not respond to glucocorticoids or when glucocorticoids are undesirable (e.g., history of laminitis, infection), azathioprine (2-3 mg/kg PO every 24 h for induction, then every 48 h for maintenance) or aurothioglucose (1 mg/kg IM every week for induction, then every 1-2 months for maintenance) may be useful (see Therapy of Immune-Mediated Skin Diseases).* Both of these agents, an omega-3/omega-6 fatty acid-containing product, or vitamin E (13 International Units/kg PO every 24 h), may be useful for reducing required dosages of glucocorticoids.12,29 Anecdotal reports indicate that pentoxifylline may be useful.12,25,29
Some horses respond to therapy and remain in prolonged remission without further treatment.† However, about 50% of these cases relapse within 2-30 months. In general, rapid relapses and multiple recurrences often become progressively less responsive to previous treatment regimens.12 Some mares requiring low-dose alternate-morning prednisone to control their pemphigus foliaceus have been successfully bred.12
Pemphigus foliaceus is often worse and more difficult to control in sunny, hot, humid weather.* Sun avoidance and insect control are very important in these instances. Gentle cleansing is often useful for decreasing the increased pruritus often associated with heat and sweating.12
Pemphigus vulgaris
In humans and dogs, the major pemphigus vulgaris antigen is desmoglein 3, a 130-kDa glycoprotein from the cadherin group of adhesion molecules.1,2 In more severe cases that also have cutaneous involvement, antibodies to desmoglein 1 are also present. The exact antigen in the horse has not been identified.
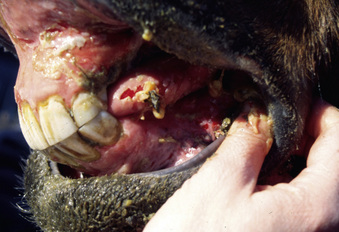
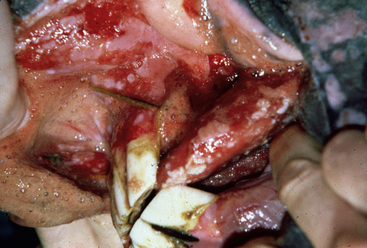
Pemphigus vulgaris is extremely rare in the horse.5,7,12,14 Vesicles, bullae, and resultant painful ulcers occur in the mouth (Fig. 9-19), on the head and neck, and at mucocutaneous junctions (Fig. 9-20). Skin biopsy specimens reveal suprabasilar acantholysis, cleft, and vesicle formation. Direct immunofluorescence testing reveals the intercellular deposition of Ig within affected epidermis.
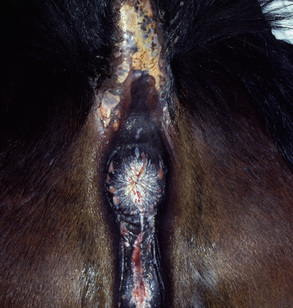
Figure 9-20 Same horse as in Fig. 9-19. Ulceration of anus, vulva, and perineum.
(Courtesy M. Sloet.)
Prognosis appears to be grave. Systemic glucocorticoid therapy has not been effective to date.
Paraneoplastic pemphigus
This form of pemphigus has been characterized in humans and dogs.1,2 The diagnostic criteria established in humans include the following: severe oral ulceration with polymorphous skin eruptions; histology that includes intraepithelial acantholysis, keratinocyte apoptosis, and vacuolar interface changes; direct immunofluorescence testing findings that include the intercellular deposition of IgG within the epithelium, and C3 in a granular pattern along the basement membrane zone; and indirect immunofluorescence findings that include IgG deposition in the intercellular spaces on monkey esophagus and rodent urinary bladder. The immunofluorescence findings on rodent urinary bladder have a specificity of 83% and a sensitivity of 75%; thus, false-positive and false-negative results occur. Immunoprecipitation studies are needed to confirm the diagnosis by identifying the characteristic antigen complex: plakins (desmoplakins 1 [250 kDa] and 2 [210 kDa], bullous pemphigoid antigen 1 [230 kDa], envoplakin [210 kDa], periplakin [190 kDa]), desmoglein 3 (130 kDa), and an unidentified (170 kDa) transmembrane antigen. Antibodies from human patients reproduce the cutaneous lesions when injected into newborn mice.
A 6-year-old Tennessee walking horse gelding had a history of painful bullae and ulcers of the tongue, gingiva, and lips, along with progressive lethargy, inappetence, and weight loss.28 These signs were accompanied by a slowly enlarging firm, round, intramuscular mass on the neck. Biopsy specimens from the oral mucosa revealed subepidermal clefts and vesicles in association with a perivascular accumulation of lymphocytes, plasma cells, and neutrophils. Direct immunofluorescence testing revealed the intercellular deposition of IgG in the epithelium. Treatment with prednisone, 2.2 mg/kg every 12 h PO, was of no benefit.
Eight weeks later the horse returned for progressive disease, and the cervical mass was excised and reported to be a hemangiosarcoma. A later publication indicated that the mass was probably a reticulum cell sarcoma.18 Within 1 week following surgery, the horse began to improve and went on to totally recover. Serum from the horse that had been frozen since the initial visit reacted with the keratinocytes in monkey esophagus and the transitional cells of murine urinary bladder. Immunoprecipitation studies demonstrated that the horse’s antibodies reacted with polypeptides at 250 and 210 kDa (desmoplakin 1 and 2), 230 kDa (bullous pemphigoid antigen 1), 210 kDa (envoplakin), 190 kDa (periplakin), and 170 kDa (uncharacterized).
Autoimmune Subepidermal Bullous Dermatoses
The classification of autoimmune blistering diseases is based on the antigen(s) targeted by pathogenic autoantibodies. The autoimmune subepidermal bullous diseases of humans and companion animals represent different nosologic entities: bullous pemphigoid, mucous membrane pemphigoid, linear IgA bullous dermatosis, epidermolysis bullosa acquisita, and bullous systemic lupus erythematosus.1,2 These entities are similar clinically, histopathologically, and with routine immunofluorescence or immunohistochemical testing. However, they are different diseases with important prognostic and therapeutic differences. A similar situation may exist with equine subepidermal bullous diseases. To date, only bullous pemphigoid has been documented in horses.
Bullous pemphigoid
Bullous pemphigoid is a very rare autoimmune, vesiculobullous, ulcerative disorder of skin or oral mucosa or both.*
Cause and pathogenesis
The first antigen is bullous pemphigoid antigen 1 (BPAg 1, BP230), which is a 230-kDa intracellular antigen that is a homolog to desmoplakin I. Serum antibodies to this antigen have not yet been described in horses. The second bullous pemphigoid antigen (BPAg 2, BP180), also called collagen XVII, is a 180-kDa hemidesmosomal transmembranous molecule. Equine cases of bullous pemphigoid exhibit antibodies to this latter molecule.1,31 The cause of antibody production is unknown. In humans, the expression of BPAg is greatest in skin where lesions commonly occur. Pemphigoid antibodies from affected humans produce locally the clinical, histologic, and immunopathologic features of bullous pemphigoid when injected into rabbit cornea, guinea pig skin, or neonatal mice.
The proposed pathomechanism of blister formation in bullous pemphigoid is as follows: (1) the binding of complement-fixing pemphigoid antibody to the antigen of the hemidesmosomes, (2) complement fixation and activation, (3) activation of mast cells and release of chemotactic cytokines, (4) chemoattraction of neutrophils and eosinophils, and (5) release of proteolytic enzymes from the infiltrating leukocytes, which disrupt dermoepidermal cohesion, resulting in dermoepidermal separation and vesicle formation.1,2
Other factors thought to be involved in the pathogenesis of some cases of bullous pemphigoid are drug provocation (especially sulfonamides, penicillins, and furosemide), UV light, and genetic predilection.1,2
Clinical features
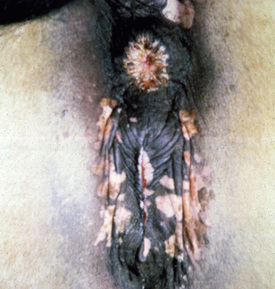
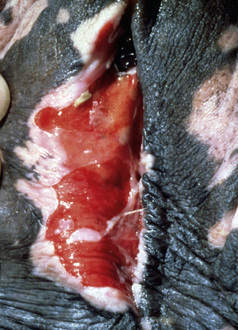
Bullous pemphigoid is very rare and accounts for only 0.2% of the equine skin diseases seen at the CUHA. It is a severe, rapidly progressive, mucocutaneous disorder.* No breed or sex predilections are apparent, and affected horses have varied in age from 5- to 14-years old. The oral cavity, mucocutaneous junctions (lips, vulva, anus, eyelids), and intertriginous areas (axilla, groin) are ulcerated, oozing, and crusted (Figs. 9-21–9-24). Epidermal collarettes are prominent, but intact vesicles and bullae are transient and not commonly seen. Lesions are painful. One horse had corneal ulcers and edema of the distal limbs and ventrum.12 Another horse had immune deposits in the kidneys.12 All horses have been severely ill (depression, anorexia, weight loss, pyrexia).
Diagnosis
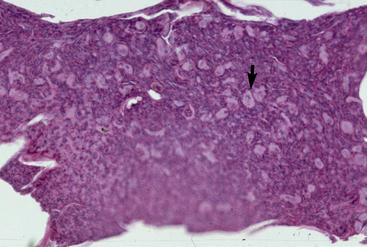
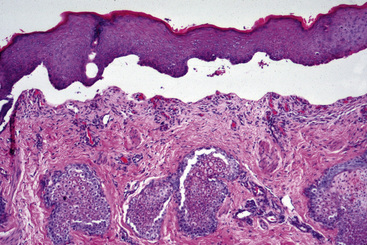
The definitive diagnosis is based on history, physical examination, skin or mucosal biopsy, immunofluorescence or immunohistochemical testing, and demonstration of the antigen targeted (BPAg2, collagen XVII). Histopathologically, bullous pemphigoid is characterized by subepidermal cleft and vesicle formation (Fig. 9-25). Acantholysis is not seen. Inflammatory infiltrates vary from mild and perivascular (cell-poor) to moderate and interstitial (cell-rich). Neutrophils and mononuclear cells usually predominate. Tissue eosinophilia has not been reported. Subepidermal vacuolar alteration is the earliest prevesicular histopathologic finding (Fig. 9-26).
Electron microscopic examination reveals the following features: smudging, thickening, and interruption of the basement membrane zone; fragmentation and disappearance of the anchoring fibrils, anchoring filaments, and hemidesmosomes; basal cell degeneration; and separation occurring within the lamina lucida.1,2
Direct immunofluorescence testing or immunohistochemical testing reveals a linear deposition of Ig, and usually complement, at the basement membrane zone of skin or mucosa.12,31 Indirect immunofluorescence testing is usually positive (IgG autoantibodies directed at the basement membrane) (Fig. 9-27).
Lupus Erythematosus
Lupus erythematosus is a term that encompasses a group of diseases with different clinical syndromes that share a similar underlying autoimmune process.2 The appropriate terminology and classification of the syndromes and cases with these disorders have led to, and still create, differing views and controversy. The terminology and a classification system used in humans is beginning to be used in veterinary medicine. In this system, the basis is that there is lupus erythematosus that may be systemic or cutaneous. The systemic form of lupus erythematosus may be associated with any of the lupus-related skin diseases or many nonspecific cutaneous lesions. The lupus erythematosus-related skin disease might be nonspecific, or it may be one of the forms of cutaneous lupus erythematosus that are specific skin syndromes, characterized by certain clinical and histopathologic findings. The different forms of cutaneous lupus erythematosus vary as concerns the probability of associated systemic disease. Two main forms of cutaneous lupus erythematosus are recognized in the horse.
Systemic lupus erythematosus is an autoimmune disorder with a complex, multifactorial, and poorly defined pathogenesis.2,13 The exact etiology is unknown, but in humans, all forms are characterized by a variety of autoantibodies to nuclear antigens and/or immune complex deposition. Genetic associations have been described in humans and dogs. In humans, the different forms of cutaneous lupus erythematosus have different genetic markers. Other precipitating factors include viral infections, drugs, hormones, chemical exposure, and cigarette smoking.
The pathogenesis of skin lesions in lupus erythematosus is unclear. All three forms of cutaneous lupus erythematosus in humans, and canine cutaneous lupus erythematosus, are often exacerbated by exposure to UV radiation.2 In humans, it has been demonstrated that the lymphocytes infiltrating skin lesions of discoid and systemic lupus erythematosus are predominantly T-cells. Five characteristics of cutaneous lupus erythematosus are: (1) photosensitivity (lesions may be produced by sunlight in both the UVB and UVA spectra), (2) keratinocyte damage (associated with contiguous T-lymphocytes and macrophages), (3) lymphohistiocytic infiltration, (4) autoantibody production, and (5) immune complex deposition.2,13 Skin lesions may be induced or exacerbated with UV light exposure. Infusion of antinuclear antibodies does not produce skin lesions, however, and immune complexes appear at the basement membrane zone after dermatohistopathologic changes appear (up to 6 weeks after inflammatory changes emerge).