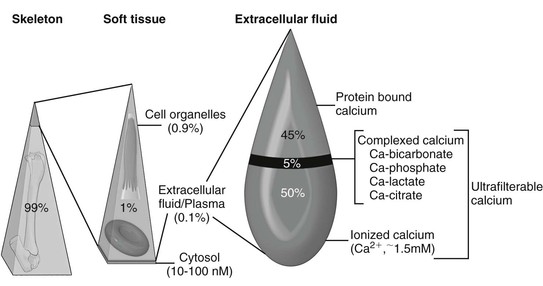
Ramiro E. Toribio Calcium is crucial to a multitude of body functions, ranging from structural support and protection of internal organs to regulatory actions. Several intracellular and extracellular processes, physiologic and pathologic, depend on calcium as a modulatory ion. Calcium is necessary for blood coagulation, neuromuscular excitability, muscle contraction, enzymatic activation, hormone secretion, cell division, cell membrane stability, and cell death. Extracellular concentrations of calcium are influenced by physiologic (e.g., pregnancy, lactation, exercise, growth) and pathologic (e.g., sepsis, gastrointestinal disease, renal injury, neoplasia) processes. Given its pleiotropic functions, keeping extracellular calcium concentrations within a narrow range is essential. In equine blood, total calcium (TCa) is made up of factions bound to proteins, complexed to anions (bicarbonate, phosphate, lactate, citrate), and circulating in a free or ionized form (Ca2+: biologically active calcium; Box 185-1; Figure 185-1). Blood TCa and Ca2+ concentrations are lower in foals than in adult horses, and Ca2+ represents a lower percentage (about 50%) of the TCa, compared with adult horses (about 55%). Calcium requirements depend on age, physiologic status, and physical activity. Requirements in neonates are higher because of rapid growth and skeletal mineralization. Similar to TCa, blood total magnesium (TMg) is bound to proteins, complexed to anions, and free or ionized (Mg2+: active). The calcium homeostatic system consists of three body systems (kidney, intestine, and bone), three hormones (parathyroid hormone [PTH], calcitonin [CT], and 1,25-dihydroxyvitamin D3 [1,25(OH)2D3; calcitriol]), and a calcium-sensing receptor (CaSR). Mg2+ is also essential in Ca2+ regulation because PTH release, PTH action, and 1,25(OH)2D3 synthesis are Mg2+-dependent processes. A decrease in extracellular Ca2+ concentrations will increase PTH secretion by the parathyroid gland, which in turn acts in the kidney to increase calcium reabsorption and 1,25(OH)2D3 synthesis, and in bone to increase osteoclastic bone resorption, with the goal of restoring normocalcemia. PTH also increases urinary excretion of phosphorus (Pi). 1,25 (OH)2D3 increases intestinal absorption and renal reabsorption of Ca2+ and Pi. In response to hypercalcemia, the C cells of the thyroid gland secrete CT to inhibit osteoclastic activity, decrease renal reabsorption of calcium, and reduce serum Ca2+ concentrations. Parathyroid hormone–related protein (PTHrP) is an autocrine or paracrine factor that activates the PTH receptor; it is important for transplacental Ca2+ transport and Ca2+ homeostasis in the fetus, but not in the adult. It is suspected that increased PTHrP or 1,25(OH)2D3 concentrations may be involved in the pathogenesis of a rare syndrome of hypercalcemia and asphyxia in newborn foals. Acidosis increases and alkalosis decreases serum Ca2+ and Mg2+ concentrations. Hypoalbuminemia will reduce TCa concentrations but has a minimal effect on Ca2+ concentrations (pseudohypocalcemia). Although hypocalcemia in adult horses is mostly associated with gastrointestinal disease, hypocalcemia in foals in general is linked to critical illness, sepsis, and gastrointestinal disorders. Hypocalcemia can have devastating consequences in the equine neonate by altering neuromuscular activity, cardiac contractility, blood pressure, tissue perfusion, and gastrointestinal function. Conditions that have been associated with hypocalcemia in foals include hypoparathyroidism, hypomagnesemia, sepsis (endotoxemia, cytokines), enteritis, enterocolitis, rhabdomyolysis, hypoalbuminemia, idiopathic hypocalcemia, pancreatitis, acute renal injury, hyperphosphatemia (cell lysis, enemas), and alkalosis (Box 185-2). Other proposed, but poorly documented, causes of hypocalcemia include intracellular shift (sequestration, chelation), hypovitaminosis D, PTH resistance (pseudohypoparathyroidism), and intestinal loss of calcium. More than one factor (e.g., inflammatory cytokines and hypomagnesemia) could be active in any given foal that further contributes to hypocalcemia. Clinical evaluation of the calcium homeostatic system requires measurement of serum TCa, Ca2+, TMg, Mg2+, Pi, PTH, total protein, and albumin concentrations. Measuring Mg2+ concentrations in foals with hypocalcemia is strongly warranted. PTHrP and CT concentrations usually provide minimal information unless there is evidence of prolonged calcium dysregulation. Assessment of the acid-base status is recommended to rule out alkalosis as a cause of ionized hypocalcemia. For converting from standard to SI units, the calcium conversion factor is as follows: 1 mg/dL × 0.25 = mmol/L; 1 mmol/L = 4 mg/dL of calcium. The term hypoparathyroidism encompasses a number of pathologic processes, congenital and acquired, that can be primary or secondary to parathyroid gland disease, that all lead to reduced PTH secretion. Hypoparathyroidism has been confirmed in foals. Primary hypoparathyroidism is the result of an inherent failure of the parathyroid gland to secrete PTH (congenital or acquired), whereas secondary hypoparathyroidism is the consequence of disease processes outside the parathyroid tissue (magnesium depletion, cytokines). In humans, various mutations are linked to primary hypoparathyroidism; however, no mutation has been associated with hypoparathyroidism in domestic animals. Autoimmune hypoparathyroidism has been reported in humans, wherein antibodies against the CaSR result in receptor activation and suppression of PTH synthesis. This phenomenon has not been demonstrated in domestic animals and seems unlikely in the neonate. Human infants born of mothers with hyperparathyroidism or diabetes may develop neonatal hypocalcemia. Presumably, the high maternal Ca2+ concentrations in hyperparathyroid mothers suppress fetal parathyroid gland function. Unthriftiness, painful joints, and soft tissue mineralization were described in a 3-week-old foal whose dam had secondary hyperparathyroidism; however, the foal did not have hypocalcemia or hypoparathyroidism. Most cases of hypoparathyroidism in foals and horses are secondary (to sepsis, cytokines, or hypomagnesemia). Hypoparathyroidism should be suspected in any foal with refractory hypocalcemia. Laboratory findings consistent with hypoparathyroidism include hypocalcemia, hyperphosphatemia, hypercalciuria, hypophosphaturia, and low or normal serum PTH concentrations. Hypomagnesemia may also be present. Mild hyperphosphatemia can be difficult to assess in foals with hypoparathyroidism because growing animals have higher Pi concentrations than adults. Severe hyperphosphatemia may contribute to hypocalcemia (precipitation) and soft tissue mineralization. Clinical signs of hypoparathyroidism are the same as those of hypocalcemia.
Hypocalcemic Disorders in Foals
Calcium Physiology
Hypocalcemic Disorders
Hypoparathyroidism
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree