Chapter 17 HYPOCALCEMIA AND PRIMARY HYPOPARATHYROIDISM
Several historical landmarks in the understanding of parathyroid physiology, maintenance of homeostasis, and calcium regulation are significant with respect to our knowledge of hypocalcemia. Rickets (hypovitaminosis D) was first described in 1645. More than 200 years later (1884), an association was made between thyroidectomy in dogs and cats and the development of clinical hypocalcemia (tetany). In 1891, Gley proved that the parathyroid glands must be removed with the thyroids to produce tetany. Shortly thereafter, administration of calcium salts following parathyroidectomy successfully prevented tetany. Almost a century later (1970), the amino acid sequence of parathyroid hormone (PTH) was determined (Tepperman, 1980). Almost 20 years later, the amino acid sequence of a parathyroid hormone–related peptide (PTHrP), produced by some cancers, was described in both humans and dogs (Weir, 1988; Broadus et al, 1988; Yates et al, 1988).
DEFINITION OF PRIMARY HYPOPARATHYROIDISM
A pair of parathyroid glands are located in close proximity to each thyroid lobe in normal individuals. Hypoparathyroidism, an uncommon endocrine disorder, develops as a result of an absolute or relative deficiency in secretion of PTH, the sole product of the four parathyroid glands. This deficiency causes various physiologic problems, with the final common pathway to clinical signs involving neurologic and neuromuscular disturbances resulting from hypocalcemia. The signs of hypocalcemia are similar, regardless of the cause (Table 17-1). Once identified, the clinician should attempt to determine the underlying pathogenesis (cause) for hypocalcemia, in order to formulate short- and long-term treatment strategies and a prognosis.
TABLE 17-1 SIGNS NOTED BY OWNERS OF DOGS WITH PRIMARY HYPOPARATHYROIDISM
Note that almost all these signs are “episodic.”
PATHOPHYSIOLOGY
Systemic Calcium Homeostasis
Maintenance of a normal serum calcium concentration
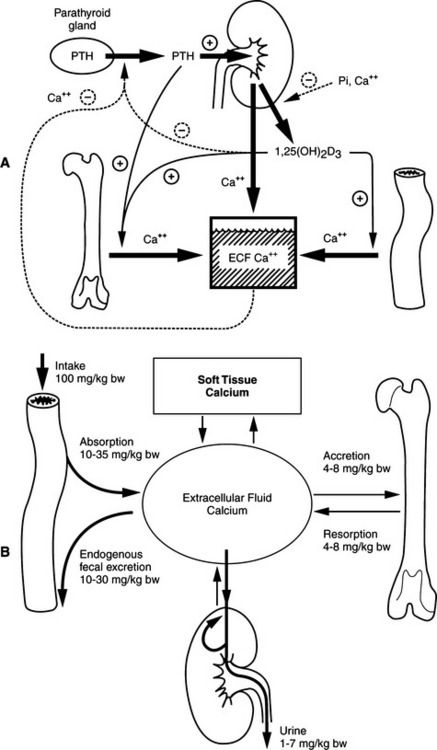
The parathyroid glands are exquisitely sensitive to small changes in the serum ionized calcium concentration. The integrated actions of PTH on calcium resorption from bone, distal renal tubular calcium reabsorption, and 1,25(OH)2 cholecalciferol (vitamin D; calcitriol)–mediated intestinal calcium absorption are responsible for the fine regulation of serum ionized calcium concentration. The precision in this inte-grated control is such that plasma ionized calcium concentrations probably fluctuate day to day by no more than 0.1 mmol/L from their “set” normal value in healthy animals. The “acute” phases of bone resorption and distal renal tubular calcium reabsorption are major control sites in minute-to-minute calcium homeostasis. The effect of PTH on the distal renal tubule is quantitatively more important. Adjustments in the rate of intestinal calcium absorption via the calcium–PTH–vitamin D axis require approximately 24 to 48 hours to become maximal (Broadus, 1981).
Defense against hypocalcemia
THE CHALLENGES.
The physiologic response to a hypocalcemic challenge can be characterized by several distinct categories of adjustment in mineral metabolism (Fig. 17-1). The classic challenges include (1) a minor, transient challenge, (2) a moderate challenge, and (3) a severe, prolonged challenge. Hypocalcemia elicits corrective homeostatic responses that are mediated by PTH and vitamin D. Acute effects occur in seconds to minutes, subacute or moderate effects occur over several hours and may last a few days, and chronic effects occur over days to weeks and longer (Rosol and Capen, 1997; Rosol et al, 2000).
MINOR, TRANSIENT CHALLENGE.
A 12- to 15-hour fast (or consumption of a diet completely deficient in calcium) in a normal mammal requires only subtle hormonal adjustments. The total quantity of calcium lost in the urine over this period is small. Negligible decreases in serum calcium concentration occur, causing a slight increase in PTH secretion. The dip in serum calcium concentration is corrected by this PTH-mediated increase in calcium reclamation efficiency from distal renal tubules and by rapid calcium resorptive responses from bone. Acute secretion of preformed PTH can maintain PTH concentrations for 1 to 1.5 hours during hypocalcemia. Hypocalcemia also decreases the proportion of PTH that is degraded in the parathyroid chief cells, making more hormone available for secretion. This effect typically occurs within 40 minutes. With increases in PTH secretion, renal calcium reabsorption and phosphorus excretion are enhanced within minutes, whereas bone mobilization of calcium and phosphate occurs over a period of 1 to 2 hours (Rosol et al, 2000). By 12 hours, only minor increases in 1,25(OH)2 vitamin D synthesis have occurred.
MODERATE CHALLENGE.
An abrupt but significant reduction in dietary calcium intake (or other causes of hypocalcemia) initiates a series of adjustments in calcium metabolism, resulting in a new steady state of PTH secretion and vitamin D secretion. Several hours of persistent hypocalcemia increases PTH secretion, which in turn stimulates the synthesis and secretion of vitamin D (calcitriol). Calcitriol then increases intestinal transport of calcium and phosphorus into the vascular space, providing an external source of calcium that complements the internally mobilized calcium derived from bone. Hypocalcemia increases transcription of both the PTH gene and PTH mRNA, enhancing the ability of chief cells to produce PTH, a process that takes place within hours (Rosol et al, 2000).
Moderate increases in the secretion rate of PTH result in (1) increased calcium reabsorption from distal renal tubules, (2) increased mobilization of calcium and phosphorus from bone, and (3) increased synthesis of 1,25(OH)2 vitamin D (calcitriol), which participates with PTH in bone resorption and increases the efficiency of calcium and phosphorus absorption from the intestine (see Fig. 17-1). The increased concentrations of circulating PTH enhance renal excretion of phosphorus, thereby compensating for the increased amounts of phosphorus mobilized from bone and absorbed from the intestine. In this new steady state, the serum calcium concentration returns to normal, the serum phosphorus concentration is unchanged or slightly reduced, and a state of mild secondary hyperparathyroidism and enhanced intestinal mineral absorption efficiency exists. The initial requirement for calcium mobilization from the skeleton is largely replaced by the enhanced absorption of calcium from the intestine.
SEVERE, PROLONGED CHALLENGE.
Significant lactation and chronic renal failure represent two examples of severe hypocalcemia-related challenges to calcium homeostasis that cannot be corrected by the processes that are stimulated to occur within minutes or hours. Assuming that the four parathyroid glands are intact and functional, the previously described sequence of events resulting from “minor transient” and “moderate” challenges caused by hypocalcemia ensue. However, continuing losses of calcium into milk associated with lactation (for example) prevents complete compensation by the usual calcium–PTH–vitamin D absorption axis. Physiologic compensation in this setting includes (1) a maximal PTH secretion rate of approximately five times normal, (2) a maximal rate of 1,25(OH)2 vitamin D synthesis, and (3) initiation of maximal “rapid” and “late” phases of bone resorption in response to the combined effects of PTH and 1,25(OH)2 vitamin D.
Over days, weeks, or even longer periods of hypocalcemia, increases in PTH secretion, beyond those already described, are achieved largely via hypertrophy and hyperplasia of parathyroid gland chief cells (Roth and Capen, 1974; Rosol et al, 2000). Hypocalcemia directly stimulates the growth of parathyroid cells. This effect occurs regardless of vitamin D metabolite concentrations (Li et al, 1998; Malloy et al, 1999, Marx, 2000). With hyperplasia of parathyroid chief cells, PTH secretion rates approach 10 to 50 times normal. These circulating concentrations of PTH result in recruitment of an increasing osteoclast population and the incorporation of substantial bone surfaces into the resorption process. In the final steady state, serum calcium concentrations are maintained at the expense of the skeleton, and significant bone losses ensue. Thus the integrity of skeletal mineral homeostasis is sacrificed in an attempt to compensate for systemic mineral deficits (Broadus, 1981).
Hypoparathyroidism (Hypocalcemia)
Initial physiologic alterations
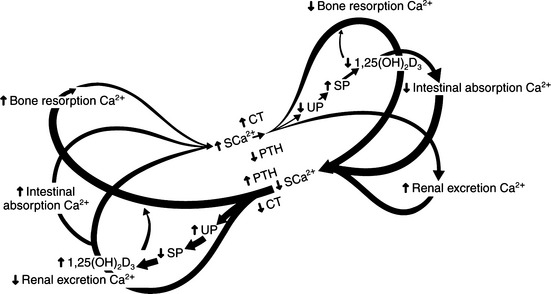
The pathologic and biochemical consequences of parathyroid gland removal (the most common cause of primary hypoparathyroidism) or loss of a critical number of parathyroid chief cells secondary to immune-mediated destruction (a less common phenomenon) can be appreciated by referring to the “butterfly” diagram (Fig. 17-2). In this condition, the right limbs of the three feedback loops predominate with (1) decreased bone resorption; (2) decreased renal phosphate excretion, increased serum phosphate, decreased calcitriol, and decreased intestinal absorption of calcium; and (3) increased renal excretion of calcium relative to the prevailing circulating concentrations of calcium. Typically, there is hypocalcemia and hyperphosphatemia, if dietary phosphate intake has been normal.
In spite of dramatic changes in the concentration of plasma calcium and phosphate, bone mineralization is normal, bone resorption rates decline, and bone formation declines only slightly. Ultimately, bones are slightly more dense than normal in humans with hypoparathyroidism, and in long-standing cases, osteosclerosis may be seen. The major signs of hypoparathyroidism are directly attributable to decreases in circulating ionized calcium concentrations, which lead to increased neuromuscular excitability.
Neuromuscular activity
PERIPHERAL NEUROMUSCULAR DISORDERS.
As discussed in Chapter 16, calcium ions are integral to the function of virtually all cells. However, although all cells in the body are affected by deficiencies in ionized calcium, clinical signs are most often associated with cells of the neuromuscular system simply because alteration in the function of these cells result in obvious visible abnormalities. Ionized calcium is involved in the release of acetylcholine during neuromuscular transmission. In addition, calcium is essential for muscle contractions and it stabilizes nerve cell membranes by decreasing their permeability to sodium. The role of calcium as a membrane stabilizer is most obvious during severe hypocalcemia, when insufficient stabilization exists.
Hypocalcemia is a relatively “common” laboratory abnormality, being observed on more than 13% of serum biochemical profiles in dogs in one report (Chew and Meuten, 1982). On the basis of total serum calcium concentration, hypocalcemia is usually defined as a concentration below 9.5 mg/dl in dogs and below 9 mg/dl in cats (Rosol et al, 2000). When serum ionized calcium concentration is used, hypocalcemia is generally defined as a concentration below 1.1 mmol/L in dogs and less than 1.0 mmol/L in cats. Clinical tetany, however, usually requires much lower serum calcium concentrations. For example, tetany can be assumed to exist whenever the serum total calcium concentration declines to or below 6 mg/dl or the serum ionized concentration to less than 0.7 mmol/L. These are values that are only 40% below normal. Total serum calcium concentrations below 4 mg/dl are frequently fatal.
When serum calcium concentrations decline to abnormal levels, but not low enough to cause obvious clinical tetany, a physical state of “latent tetany” may exist. This condition is described as one in which an individual can progress from appearing clinically normal to becoming “tetanic” with minimal stimulation. Such a condition can be demonstrated to be present in people by weakly stimulating a nerve and observing an abnormal response (see Physical Examination, page 723). If a human with latent tetany hyperventilates, the resulting alkalinization of the body fluids can increase nerve irritability, causing overt signs of tetany. It is assumed that a similar situations develop in hypocalcemic dogs or cats.
In hypocalcemic pet dogs, latent tetany occurs, but the problem is less well documented. Owners mention that sudden excitement, activity, or petting may unpredictably cause muscle cramping, lameness, facial rubbing, pain, irritability, or aggressive behavior. These signs usually disappear quickly, only to recur sporadically. In addition, the nontetanic severely hypocalcemic pet is usually described by the owner as having a change in personality. These dogs are often observed to have poor appetites and to be irritable, nonplayful, and slow-moving. Frequently, owners report that the dogs “seem to be in pain.” Such signs are vague, but after hypocalcemia is diagnosed, the clinical signs are consistent with those of an animal in latent tetany. The various disturbances are completely and quickly reversible with therapy.
THE HEART.
In experimental animals, severe decreases in serum calcium concentration can result in marked dilatation of the heart, changes in cellular enzyme activities, and in increased membrane permeability in cells outside the nervous system. Calcium has both positive inotropic and chronotropic cardiac effects (Milnor, 1980). Hypocalcemia prolongs action potential duration in cardiac cells. This may result in decreased force of myocardial contraction (negative inotropic effect) and, in severe cases, bradycardia (negative chronotropic effect). An associated increase is seen electrocardiographically in the duration of the S-T and Q-T segments. The duration of the T wave itself is not altered with hypocalcemia. Although these disturbances in physical findings and the electrocardiogram (ECG) can be pronounced in humans, they are much less obvious in the hypocalcemic dog (Kornegay et al, 1980; Sherding et al, 1980). Rarely, hypocalcemia can cause a 2:1 heart block or heart failure requiring digitalis and diuretics in humans (Arnaud and Kolb, 1983).
CLINICAL FEATURES OF NATURALLY OCCURRING HYPOPARATHYROIDISM IN DOGS
Signalment
Review of the records of 37 dogs we have seen with naturally occurring primary hypoparathyroidism (includes Bruyette and Feldman, 1988) and of reports of an additional 13 dogs with hypoparathyroidism obtained from the veterinary literature (Table 17-2) reveals several characteristics of the syndrome. Hypoparathyroidism occurs at any age, the youngest dog being 6 weeks and the oldest being 13 years (Fig. 17-3). The average age was 4.8 years. Of the 50 dogs, 30 (60%) were female. The breeds most frequently identified as having primary hypoparathyroidism were Toy Poodles, Labrador Retrievers, Miniature Schnauzers, German Shepherds, and Terriers).
TABLE 17-2 BREEDS IDENTIFIED AS HAVING PRIMARY, NATURALLY OCCURRING HYPOPARATHYROIDISM
| Breed | Number of Dogs (Total = 50)* |
|---|---|
| Toy Poodle | 10 |
| Labrador Retriever | 6 |
| Miniature Schnauzer | 5 |
| German Shepherd dog | 5 |
| Terrier breeds | 5 |
| Beagle | 2 |
| Dachshund | 2 |
| Golden Retriever | 2 |
| English Pointer | 1 |
| Collie | 1 |
| Keeshond | 1 |
| Cocker Spaniel | 1 |
| Malamute | 1 |
| Irish Wolfhound | 1 |
| Siberian Husky | 1 |
| Mixed breed | 6 |
* Includes 37 dogs from our series and 4 dogs from Kornegay et al, 1980; 6 dogs from Sherding et al, 1980; 1 dog from Meyer and Tyrrell, 1976; 1 dog from Burk and Schaubhut, 1975, and 1 dog from Crawford and Dunstan, 1985.
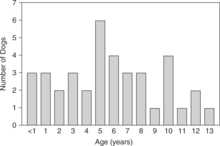
FIGURE 17-3 The ages of 38 dogs at the time of primary hypoparathyroidism diagnosis. These include 25 dogs in our series, as well as 4 dogs from Kornegay et al, 1980; 1 dog from Crawford and Dunstan, 1985; 6 dogs from Sherding et al, 1980; 1 dog from Meyer and Tyrrell, 1976; and 1 dog from Burk and Schaubhut, 1975.
Anamnesis
DURATION OF ILLNESS.
The clinical course in the 37 dogs of our series consisted of an abrupt onset of intermittent neurologic or neuromuscular disturbances (see Table 17-1). In at least 20 dogs, owners noted that signs were initiated or worsened by excitement or exercise. The signs associated with hypocalcemia had been observed for periods of 1 day in some dogs to as long as 12 months in others. Only 8 of the 37 dogs had signs for longer than 14 days before the diagnosis was made and therapy initiated. The 8 dogs with prolonged illness had been symptomatic for 1 to 12 months and had been diagnosed and treated for nonspecific seizure disorders without the benefit of pretreatment laboratory testing. The dogs with signs for more than several days invariably had neuromuscular disturbances that became progressively more frequent and violent despite administration of anticonvulsant medication.
SEIZURES.
Grand mal convulsions were observed by the owners in 32 of our 37 dogs with naturally occurring primary hypoparathyroidism. As previously reported, most of these dogs had typical grand mal convulsions. However, some seizures were atypical, in that the dogs either did not appear to lose consciousness or were not incontinent during the episode (Peterson, 1986). Of interest was the incidence of seizure activity seen by veterinarians. Of the 37 dogs, 32 were observed by a veterinarian to have seizures (often being suspected of having idiopathic epilepsy) or to be “in tetany.” This represents a much higher incidence of veterinarian-witnessed neuromuscular disorders than expected with idiopathic epilepsy. The neuromuscular problems became so severe that several dogs, although not having active obvious seizure activity, were not able to stand or walk. Also, as noted by other investigators (Sherding et al, 1980), the muscle tremors during some episodes began in one limb and gradually became generalized and progressively more violent, finally culminating in a generalized seizure. In some dogs, seizure episodes were as brief as 30 to 90 seconds; in others, they lasted for more than 30 minutes. Most, but not all, of the generalized seizures spontaneously abated.
MISCELLANEOUS SIGNS.
As can be seen in Table 17-1, many owners observed overlapping neurologic and neuromuscular signs. It can be safely stated, retrospectively, that each dog suffered bouts of significant hypocalcemic tetany as a part of their initial signs. Some vague signs included panting, ataxia, episodic weakness, complete anorexia, vomiting, diarrhea, and weight loss. Veterinarians noted fever, a problem not observed by owners. Previously described signs of circling and/or disorientation (Sherding et al, 1980) were not observed in our group of 37 hypocalcemic dogs. All of our owners observed some clinical signs; failure to see any sign, reported by others, was not noted. Although hypocalcemia was almost always considered a serendipitous finding on laboratory testing, it remains an abnormality that “made sense” after being demonstrated. Death remains a potential sequela of untreated hypocalcemia but is quite uncommon.
FACIAL RUBBING.
Of the 37 dogs in our series, 23 were observed to paw or rub violently at their muzzles, eyes, and ears and to rub their muzzles on the ground. Alternatively, owners noted their dogs intensely licking or chewing at their paws. These signs are thought to result from the pain associated with masseter and temporal muscle cramping caused by the hypocalcemia or as a result of the “tingling” sensation around the mouth or at the distal extremities. Classic signs of hypocalcemia in humans include “paresthesias,” defined as numbness and tingling that often occur around the mouth, in the tips of fingers, and sometimes in the feet (Arnaud, 1994). Dogs, assuredly, suffer these same sensations, which may account for facial rubbing as well as biting of their feet (see Table 17-1).
HYPERVENTILATION.
Because of the acute anxiety or pain associated with tetany, hypoparathyroid humans (and presumably dogs) may episodically hyperventilate and secrete increased amounts of epinephrine. Hyperventilation causes hypocapnia and alkalosis, which worsen hypocalcemia by causing increased binding of ionic calcium to plasma proteins. Prolonged hyperventilation in normal human subjects can cause a decrease in the serum ionic calcium concentration. It is almost impossible, however, for tetany to be caused solely by hyperventilation (Arnaud and Kolb, 1983).
EPISODIC NATURE OF THE ILLNESS.
All neurologic and neuromuscular signs in our hypocalcemic dogs were episodic in nature. Episodes of apparent or confirmed hypocalcemic tetany were followed by asymptomatic periods. The periods of clinical well-being lasted minutes to days. Tetany was rather unpredictable, although retrospectively, these signs were much more frequent or inducible with exercise (even slow or short “leash” walks), excitement, petting (“latent tetany”?), or stress (being taken to the veterinarian).
Physical Examination
GENERAL OBSERVATIONS.
Other than signs related to hypocalcemia, dogs with primary hypoparathyroidism do not have other abnormalities on physical examination. Findings on physical examination performed on the 37 hypoparathyroid dogs varied (Table 17-3). Numerous dogs were in tetany on presentation. Most of the time this was an observation made after serum biochemical results were reviewed. Alternatively, 33 of the 37 dogs were referred for evaluation of hypocalcemia and the veterinarian who examined the dog initially (usually one of us) was “primed” to observe tetany. These observations (almost all of which are noted in Table 17-3), although impressive to the uninitiated, may not have been made had the history not alerted us to the underlying condition.
TABLE 17-3 INITIAL PHYSICAL EXAMINATION FINDINGS IN 37 DOGS WITH NATURALLY OCCURRING PRIMARY HYPOPARATHYROIDISM
| Sign | Number of Dogs | |
|---|---|---|
| Seizure or “in tetany” | ||
| Initial examination | 18 | |
| In first 4 days of hospitalization | 32 | |
| Fever | 26 | |
| Tense, splinted abdomen | 24 | |
| Stiff gait | 23 | |
| Thin | 22 | |
| Generalized muscle fasciculations | 21 | |
| Growling | 21 | |
| Cardiac abnormalities | ||
| Tachyarrhythmia | 13 | |
| Muffled heart sounds/weak pulse | 3 | |
| Neurologic examination difficult to complete and interpret | 30/30 | |
| Cataracts | 12 | |
| No abnormality | 5 | |
INDUCED NEUROLOGIC OR NEUROMUSCULAR SIGNS.
Two physical tests are used in humans as aids in diagnosing latent tetany (hypocalcemia). Chvostek’s sign is elicited by tapping the facial nerve just anterior to the ear lobe. A positive sign is one of extensive facial muscle twitching or muscle contraction. Trousseau’s sign is induced with a blood pressure cuff inflated above systolic blood pressure for at least 2 minutes. A positive response consists of carpal spasm, at least 5 to 10 seconds in duration, after release of the cuff or while the cuff is inflated (Arnaud, 1994; Meininger and Kendler, 2000). Although not well described in the dog, episodes of intense muscle spasm have been stimulated when testing reflexes in hypocalcemic dogs.
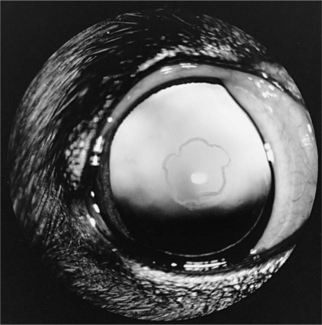
CATARACTS.
Cataracts were seen in 12 of the 37 dogs in our series and were first reported in 2 hypoparathyroid dogs in 1980 (Kornegay et al, 1980). Similar cataracts have been reported in several cats and were noted in 2 of the 5 cats with primary hypoparathyroidism in our series. These cataracts have been small punctate to linear white opacities in the anterior and posterior cortical subcapsular region. The opacities are randomly distributed along the lens fibers and are separated from the capsule by an intervening zone of normal thin cortex (Fig. 17-4). There has been no loss of vision. Other ocular signs not yet reported in dogs include papilledema, optic neuritis, conjunctivitis, keratitis, blepharospasm, loss of lashes, strabismus, nystagmus, and anisocoria.
CLINICAL FEATURES OF NATURALLY OCCURRING HYPOPARATHYROIDISM IN CATS
The clinical features of the 14 cats with naturally occurring hypoparathyroidism are much like those reported in humans and dogs except that a majority of the cats (9) have been male. The cats were 6 months to 7 years of age at the time of diagnosis and several breeds were represented. The clinical course of each cat was characterized by an abrupt or gradual onset of intermittent neurologic or neuromuscular disturbances, which included focal or generalized muscle tremors, seizures, ataxia, stilted gait, disorientation, and weakness. Other commonly observed abnormalities included lethargy, anorexia, panting, and raised nictitating membranes. Less commonly, dysphagia, pruritus, and ptyalism were observed by their owners. Physical examination findings included depression, weakness, fever, hypothermia, bradycardia, and mild to severe dehydration. Lenticular cataracts were detected in several of these cats (Forbes et al, 1990; Parker, 1991; Peterson et al, 1991; Bassett, 1998; Ruopp, 2001).
DIAGNOSTIC EVALUATION: ROUTINE STUDIES
Calcium
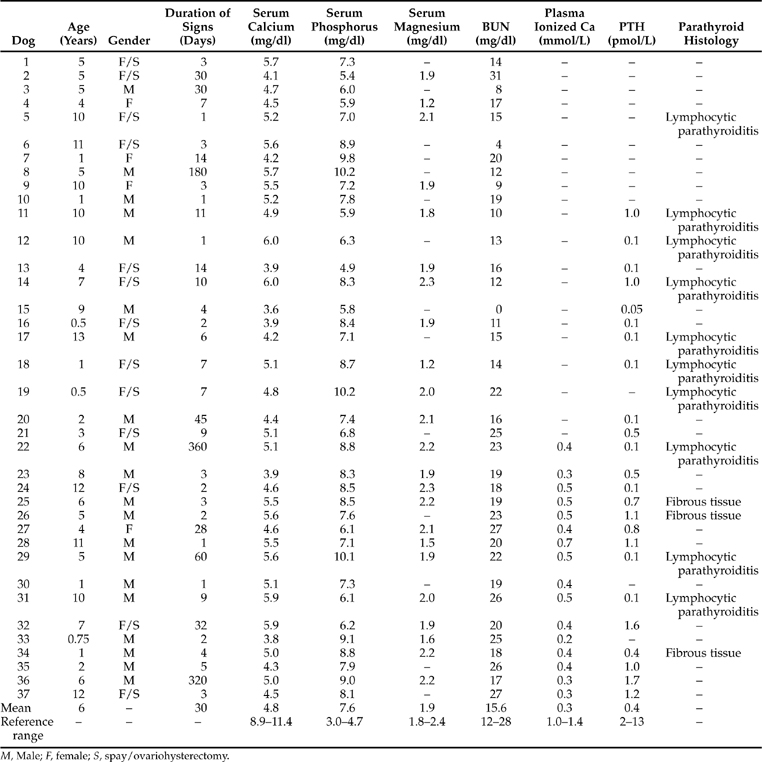
Hypocalcemia was a serendipitous finding in each of our 37 dogs with primary, naturally occurring hypoparathyroidism. Each dog had a history consistent with a behavioral, neurologic, muscular, or neuromuscular disorder. Therefore a database consisting of a complete blood count, urinalysis, and serum chemistry profile was deemed necessary in the evaluation of each dog. Each dog was severely and persistently hypocalcemic (Table 17-4). Since severe hypocalcemia (serum calcium concentration <6.5 mg/dl) is an unusual finding in our clinic population, this parameter was invariably rechecked with a separate blood sample. Then, because therapy for hypocalcemia was quickly instituted, each dog had its serum calcium concentration monitored three to five times during the first 72 hours of hospitalization. In no dog was the serum calcium concentration greater than 6.5 mg/dl until therapy began to have an effect. Since ionized plasma concentrations became routinely available, these values were also assessed. As can be noted from results reported in Table 17-4, each dog with primary hypoparathyroidism was profoundly deficient in the ionized fraction of calcium, as well as being deficient in the total fraction of calcium.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree