Chapter 8 HYPOADRENOCORTICISM (ADDISON’S DISEASE)
BACKGROUND
The presence of the “suprarenal glands” was recognized by early anatomists, but their importance was not apparent until Thomas Addison described a clinical syndrome in humans that he associated with their dysfunction (Addison, 1855). Included in his description were “anemia, general languor, debility, remarkable feebleness of the heart’s action, and irritability of the stomach.” Autopsies usually revealed either tuberculous destruction or atrophy of the adrenal glands. At that time, no therapy was known, and patients who developed the disease died. About the same time that Thomas Addison described the clinical picture of adrenal insufficiency, Brown-Sequard (1856) demonstrated that adrenalectomy resulted in death in experimental animals, thus documenting the necessity of the adrenal glands for maintaining life.
Synthetic desoxycorticosterone acetate (DOCA) was shown to be of benefit in the maintenance therapy of patients with adrenal insufficiency by Thorn and his co-workers in 1942. The beneficial effects of salt and desoxycorticosterone administration were related to correction of electrolyte disturbances and associated dehydration. Although these treatments were helpful for patients with partial adrenal insufficiency, they did not provide protection from severe stress (Nelson, 1980).
ETIOLOGY
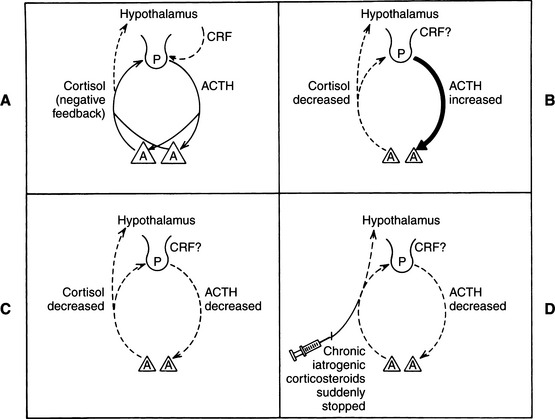
Hypoadrenocorticism is a syndrome that usually results from disease affecting both adrenal cortices (Fig. 8-1). Loss of more than 85% to 90% of adrenocortical cells appears to be required before clinical signs of deficient glucocorticoid and mineralocorticoid secretion (primary adrenocortical failure) become obvious. Less commonly, abnormalities in the hypothalamic-pituitary axis can result in reduced secretion of the “trophic” hormone, adrenocorticotropic hormone (ACTH). Loss of ACTH has the potential to cause atrophy of the adrenal cortices (sparing the zona glomerulosa) and impaired secretion of glucocorticoids (secondary adrenocortical failure). Isolated hypoaldosteronism is a recognized but rare syndrome in humans usually due to inadequate secretion by the kidneys. Reninemic hypoaldosteronism without glucocorticoid deficiency has been described in a dog that also had a heart base chemodectoma (Lobetti, 1998).
Primary Adrenocortical Failure
IMMUNE-MEDIATED (AUTOIMMUNE) DISEASE.
Idiopathic adrenal insufficiency is the most common diagnostic “label” for dogs with adrenocortical failure, because the cause of the disease is usually not obvious. However, the disease is most common in young to middle-age female dogs, and many of the features of the disease resemble those in humans. The most common cause of human hypoadrenocorticism is immune-mediated destruction of the adrenal cortices (Table 8-1), which affects four times as many women as men (Findling and Tyrell, 1991). In the active phase of the disease, histologic examination of the adrenals reveals widespread but variable infiltrates consisting of lymphocytes, plasma cells, and macrophages. In advanced stages, the cortex is replaced by fibrous tissue (Findling et al, 1997).
TABLE 8-1 CAUSES OF PRIMARY AND SECONDARY ADRENAL INSUFFICIENCY IN HUMANS
| Primary Adrenal Insufficiency | Secondary Adrenal Insufficiency |
|---|---|
| Slow Onset | |
| Abrupt Onset | |
| Adrenal hemorrhage, necrosis, or thrombosis in meningococcal or other kinds of sepsis, in coagulation disorders or as a result of warfarin therapy, or in antiphospholipid syndrome | |
* Type I autoimmune polyglandular syndrome consists mainly of adrenal insufficiency, hypoparathyroidism, and mucocutaneous candidiasis. Type II autoimmune polyglandular syndrome consists mainly of adrenal insuffi-ciency, autoimmune thyroid disease, and insulin-dependent diabetes mellitus.
† Diabetes insipidus is often present.
From Oelkers (1996); used with permission.
It is likely that most dogs and cats with naturally occurring hypoadrenocorticism also have immune-mediated destruction of the adrenal cortices (Schaer et al, 1986; Reusch, 2000). Idiopathic atrophy of all the layers of the adrenal cortex continues to be the most frequently observed histologic lesion in dogs with hypoadrenocorticism. Necropsy of recently afflicted dogs is not common, which decreases the possibility of visualizing an active inflammatory process. Most dogs afflicted with hypoadrenocorticism either die without necropsy or are diagnosed and treated. Dogs and cats treated for any length of time have no immune-mediated infiltrates and are left with atrophied/fibrotic glands. The pituitary gland is normal in primary immune-mediated adrenocortical atrophy (Boujon et al, 1994).
Humans who would formerly have been considered to have idiopathic adrenal insufficiency can now be evaluated for the presence of antiadrenocortical antibodies. In this way, immune-mediated destruction of human adrenal cortices can be confirmed with readily available laboratory testing. Similar tests have not yet been applied and reported from a large series of dogs with the syndrome, although antiadrenal antibody testing is occasionally mentioned (Kooistra et al, 1995).
MULTIPLE IMMUNE-MEDIATED DISORDERS
Humans.
Immune-mediated destruction of the adrenal glands in humans is commonly associated with other immune disorders. Two distinct immunoendocrinopathic syndromes that involve the adrenal glands in humans, autoimmune polyglandular disease type I and type II, have been described. Type I disease, typically an autosomal recessive disorder, usually begins during childhood; it involves adrenal insufficiency, hypoparathyroidism, and chronic mucocutaneous candidiasis. The more common type II disease, also called Schmidt’s syndrome, involves adrenal insufficiency, thyroiditis, and insulin-dependent diabetes mellitus (see Chapter 3). Ovarian failure occurs in both syndromes. Alopecia, malabsorption syndromes, chronic hepatitis, vitiligo, and pernicious anemia are also associated with autoimmune hypoadrenocorticism. One or more of these associated disorders are found in 40% to 53% of humans with Addison’s disease (Baxter and Tyrrell, 1981; Oelkers, 1996).
The incidence of circulating antibodies to various endocrine organs and other tissues is greater than that of overt clinical disease (Table 8-2). Enzymes involved in steroidogenesis are target autoantigens in autoimmune Addison’s disease. Of the three enzymes (17α-hydroxylase, 21α-hydroxylase, and the side-chain cleavage enzyme), 21α-hydroxylase appears to be the most important autoantigen in isolated Addison’s disease, as well as in the polyglandular syndromes (Chen et al, 1996; Soderbergh et al, 1996; Reusch, 2000). These findings suggest the potential for a genetic component in the pathogenesis of polyglandular failure. In our series of 187 dogs with primary adrenocortical insufficiency, 28 have had at least one other endocrinopathy. These problems included hypothyroidism in 16 dogs, diabetes mellitus in 14, hypoparathyroidism in three, and azoospermia in two (several dogs had more than one associated disorder). The incidence of hepatopathies has been much more significant in our hypoadrenal population (see page 415). Also of interest are dogs with naturally occurring hypoadrenocorticism and lymphocytic/plasmacytic (immune mediated? autoimmune?) gastrointestinal disorders and/or renal glomerular pathology (immune complex glomerulonephropathy).
TABLE 8-2 INCIDENCE OF CIRCULATING AUTOANTIBODIES IN HUMANS WITH AUTOIMMUNE ADRENOCORTICAL INSUFFICIENCY*
| Cell Type | Percentage | |
|---|---|---|
| Adrenal | 64 | |
| Thyroid | ||
| Cytoplasm | 45 | |
| Thyroglobulin | 22 | |
| Stomach | ||
| Parietal cells | 30 | |
| Intrinsic factor | 9 | |
| Parathyroid | 26 | |
| Gonad | 17 | |
| Islet cell | 8 | |
* Adapted from Tyrrell JB, et al: Glucocorticoids and adrenal androgens. In Greenspan FS (ed): Basic and Clinical Endocrinology, 3rd ed. Philadelphia, WB Saunders Co, 1991, p 323.
Autoimmune polyglandular disease has been documented in a few veterinary cases (Bowen et al, 1986; Kooistra et al, 1995). Any endocrine deficiency syndrome may occur secondary to immune-mediated (autoimmune) destruction. It remains to be proved whether true immune-mediated disease accounts for a significant percentage of the dogs with hypoadrenocorticism, whether there is a familial association, and whether those dogs are predisposed to other immune-mediated disorders.
OTHER CAUSES OF PRIMARY HYPOADRENOCORTICISM.
Uncommon (rare?) causes of canine adrenocortical insufficiency include destruction of the adrenal cortices by granulomatous diseases such as histoplasmosis and blastomycosis; hemorrhagic infarctions (secondary to trauma, warfarin-type toxicity, or other coagulopathies), metastases of cancer to the adrenal glands; amyloidosis of the adrenal cortices; trauma (accidents); and iatrogenic causes (surgical removal of the adrenals, rapid withdrawal of a dog from chronic glucocorticoid therapy, or overdose with the adrenocorticolytic drug o,p′-DDD, which is used in the treatment of hyperadrenocorticism). Interestingly, in humans with hypoadrenocorticism secondary to hemorrhage or metastases to the glands, bilateral adrenal enlargement (sometimes massive enlargement) is often detected with ultrasonography or computed tomography (Tyrrell et al, 1991).
Secondary Adrenocortical Failure
NATURALLY OCCURRING DISEASE.
In addition to disease processes specifically affecting the adrenal glands, reduced secretion of ACTH by the pituitary gland results in decreased synthesis and secretion of adrenocortical hormones, especially glucocorticoids (see Fig. 8-1). Reduced secretion of corticotropin-releasing hormone (CRH) by the hypothalamus may also result in secondary adrenocortical failure. Destructive lesions in the pituitary or hypothalamus that would result in ACTH or CRH insufficiency (or both) are usually caused by neoplasia; inflammation and trauma are less common causes (Velardo et al, 1992; Thodou et al, 1995) (see Table 8-1). Among humans with secondary disease, especially those with space-occupying lesions, few have only adrenal insufficiency. Other hormonal systems are usually involved, and neurologic or ophthalmologic symptoms may accompany, precede, or follow adrenal insufficiency (Vance, 1994).
IATROGENIC CAUSES
General.
Adrenal insufficiency secondary to exogenous corticosteroid administration is seen commonly in small animal veterinary practice, although it only rarely results in clinical signs (see Fig. 8-1). Any pet chronically receiving amounts of corticosteroids sufficient to suppress the hypothalamic-pituitary axis is susceptible to secondary adrenal atrophy. Adrenal suppression can occur within a few days of administration of ACTH-inhibiting doses of corticosteroids, although suppression is markedly variable among individuals. This individual variation is reflected in the fact that some dogs quickly develop iatrogenic Cushing’s syndrome from relatively low doses, whereas others show no effect from higher doses. If suppression is demonstrated, adrenal function usually recovers gradually (over a period of weeks) after hormone administration is stopped, perhaps taking more time if long-acting depot forms of glucocorticoids were used (see Table 8-2).
Glucocorticoids that Cause Suppression.
A wide variety of glucocorticoids are used in veterinary practice. It must be remembered that any glucocorticoid can suppress pituitary secretion of ACTH, potentially leading to adrenocortical atrophy. Such effects usually follow chronic glucocorticoid administration, regardless of the specific drug or route. Pituitary suppression has been documented not only with injectable and oral glucocorticoids, but also with topical dermatologic, ophthalmic, and otic preparations (Roberts et al, 1984; Moriello et al, 1988; Murphy et al, 1990). Pituitary suppression caused by glucocorticoids can also occur in cats. Furthermore, cats also may suffer adrenocortical atrophy after receiving megestrol acetate (progestogens) (Chastain et al, 1982).
Although estimates of the relative biologic effectiveness of the clinical analogs vary, studies have shown prednisone/prednisolone to be five times more potent than cortisol in suppressing ACTH secretion, and dexamethasone to be 50 to 150 times more potent. Relatively small dosages of dexamethasone, therefore, may be sufficient to produce adrenal atrophy. The long-acting “depot” injectable corticosteroids (e.g., betamethasone), however, are the most potent drugs used in small animal practice for suppressing both the pituitary-adrenal axis and the immune system. One injection of such long-acting agents has been shown to suppress the pituitary-adrenocortical axis of dogs for as long as 5 weeks (Kemppainen et al, 1981 and 1982).
How Long to Wait before Testing the Pituitary-Adrenal Axis.
Fortunately, most dogs receiving glucocorticoids for any length of time do not develop any problems after cessation of therapy. The results of an ACTH stimulation test can be used to demonstrate iatrogenic suppression of the pituitary-adrenal axis. To avoid confusion in test result interpretation or suppression of cortisol secretion associated with drug therapy, it is wise to wait at least 2 weeks but sometimes as long as 2 months after discontinuation of glucocorticoid medications before performing an ACTH stimulation test (Moore and Hoenig, 1992).
PATHOPHYSIOLOGY
Background
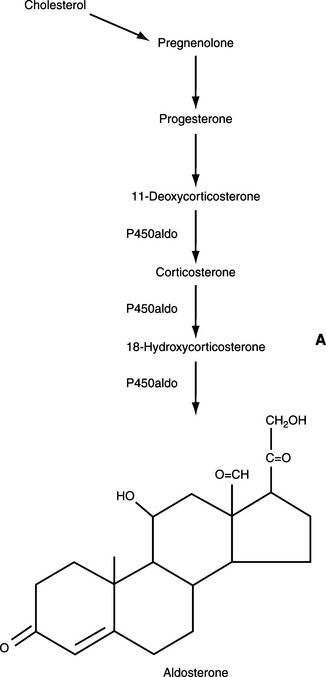
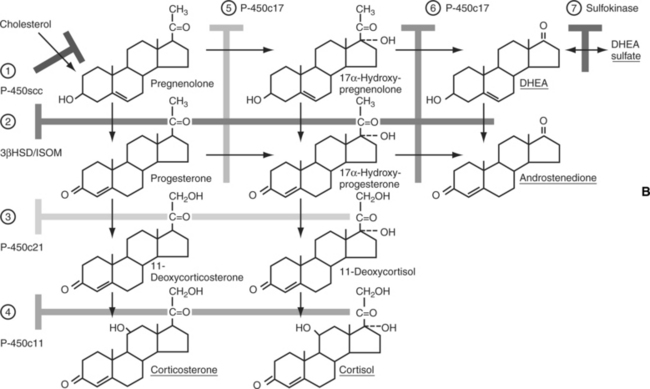
From a physiologic viewpoint, the adrenal cortices are composed of two important functional zones. The outer zone, the zona glomerulosa, synthesizes and secretes aldosterone and is under the primary control of angiotensin. The inner zone synthesizes and secretes glucocorticoids. This zone is actually composed of two histologically distinct areas, the zona fasciculata and the zona reticularis (Fig. 8-2) (Reusch, 2000).
Mineralocorticoids
PHYSIOLOGIC ACTIONS.
Mineralocorticoids function to control sodium, potassium, and water homeostasis. They promote sodium, chloride, and water resorption, as well as potassium excretion in epithelial tissues, including the intestinal mucosa, salivary glands, sweat glands, and kidneys. The primary site of aldosterone effect is the renal tubule, where aldosterone promotes proximal convoluted renal tubular resorption of sodium and chloride and distal convoluted tubular resorption of sodium by exchange with potassium (Nelson, 1980).
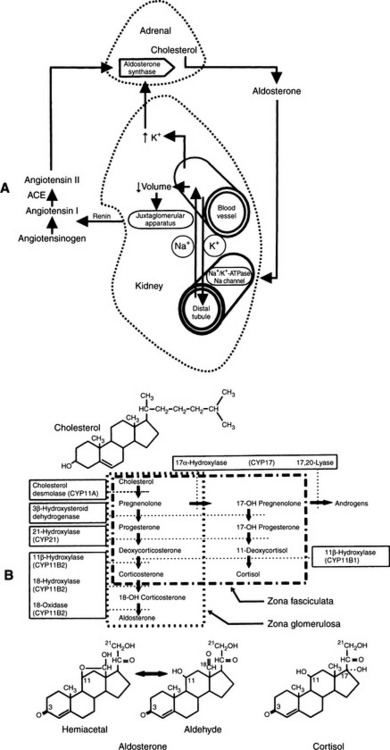
RENIN-ANGIOTENSIN SYSTEM.
Renin, in turn, acts on a plasma α2-globulin produced by the liver, releasing the decapeptide angiotensin I. Converting enzyme in the lung splits off two amino acids from angiotensin I, producing angiotensin II, which is a potent vasoconstrictor and a primary stimulant for aldosterone secretion. Increased plasma aldosterone concentrations enhance sodium retention, thereby expanding the extracellular fluid volume, increasing renal perfusion, and suppressing the initiating signal for release (Fig. 8-3) (Ganong, 1981; White, 1994).
NORMAL EFFECT OF ACTH.
The release of aldosterone can be stimulated by ACTH, but ACTH is not the dominant force in stimulation of secretion of mineralocorticoids by the zona glomerulosa. Apparently, ACTH is not important in most physiologic conditions, but rather exerts a “permissive” influence over aldosterone secretion (Tyrrell et al, 1991).
PHYSIOPATHOLOGY OF MINERALOCORTICOID DEFICIENCY
Hyponatremia and Hypochloremia.
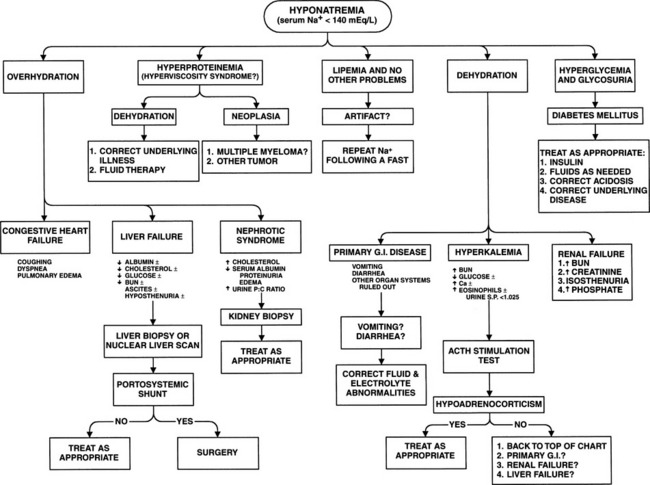
With adrenocortical insufficiency, lack of aldosterone secretion results in impaired ability to conserve sodium and chloride and to excrete potassium, leading to hyponatremia, hypochloremia, and hyperkalemia. With an adequate sodium-chloride intake, mild aldosterone deficiencies may have few if any consequences. However, if sodium intake diminishes with the onset of anorexia or a change in diet or if sodium loss increases because of vomiting and/or diarrhea, the animal’s health may quickly deteriorate. Continued loss of sodium and chloride through the gastrointestinal tract and kidneys may lead to severe depletion of total body salt stores and, simultaneously, severe volume depletion.
Antidiuretic Hormone and Urine Concentration.
The hypotension and dehydration that develop secondary to aldosterone deficiency stimulate secretion of pituitary vasopressin (AVP; antidiuretic hormone [ADH]) as the system attempts to compensate for fluid losses. Vasopressin causes water retention, potentially worsening the hyponatremia and hypochloremia by dilution. This physiologic process is not considered a major factor in hypoadrenocorticism, because these dogs tend to have relatively dilute urine (urine specific gravity of 1.010 to 1.025) despite dehydration and prerenal azotemia. Hyponatremia contributes to the development of dilute urine because sodium is an important component of the renal medullary concentration gradient. Furthermore, hyponatremia probably interferes with stimulation of ADH secretion by reducing serum osmolality. Thus decreases in sodium (and chloride) ions impair natural osmotic stimuli and promote dilute urine despite the dehydration (Tyler et al, 1987).
Hyperkalemia.
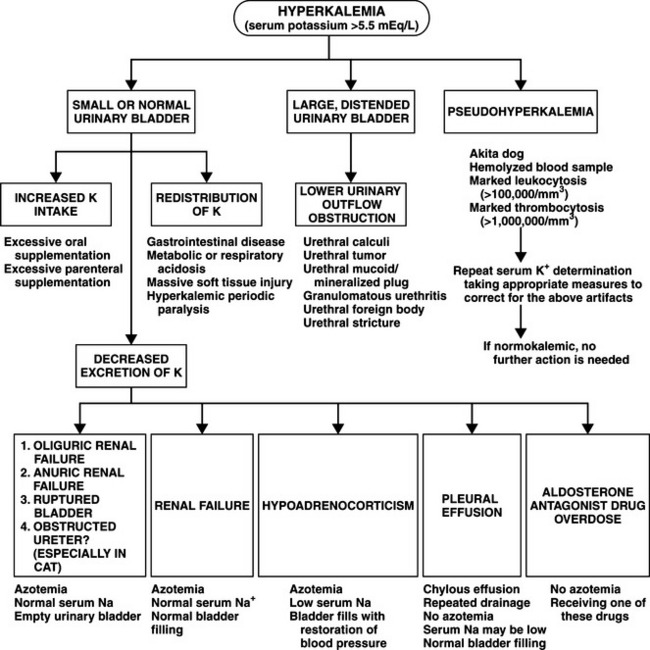
Progressively worsening hyperkalemia develops as a result of diminished renal perfusion, which reduces glomerular filtration and depresses cation exchange by the distal convoluted renal tubules. Hyperkalemia can be exaggerated by metabolic acidosis, which promotes a shift of potassium ions from the intracellular to the extracellular space. The most prominent manifestation of hyperkalemia is the deleterious effect this anion has on cardiac function. Hyperkalemia causes decreased myocardial excitability, an increase in the myocardial refractory period, and slowed conduction. Hypoxia, which occurs secondary to hypovolemia and poor tissue perfusion, contributes to myocardial irritability. These abnormalities may be demonstrated on an electrocardiogram (see page 417). Ventricular fibrillation or cardiac standstill may eventually occur as the plasma potassium concentration exceeds 10 mEq/L.
Glucocorticoids
BACKGROUND: PHYSIOLOGIC ACTIONS.
Glucocorticoid secretion is controlled by the hypothalamic-pituitary axis via a simple negative feedback loop. CRH is synthesized and secreted by the hypothalamus, and it then stimulates the secretion of ACTH by the pituitary gland. ACTH stimulates the synthesis and secretion of adrenal glucocorticoids. As plasma concentrations of glucocorticoids increase, they exert negative feedback on the secretion of CRH and ACTH (see Fig. 8-1) (Ganong, 1981). A major physiologic factor that influences the secretion of ACTH is cortisol metabolism, which reduces negative feedback and releases CRH. A second major factor in ACTH release results from stress (Nelson, 1980). Diurnal fluctuation of ACTH secretion, considered a well-documented phenomenon in humans, is not well documented in dogs.
Glucocorticoids (cortisol) affect almost every tissue in the body. Cortisol has a vital supportive role in the maintenance of vascular tone, endothelial integrity, vascular permeability, and the distribution of total body water in the vascular compartment (Lamberts et al, 1997). Cortisol potentiates the vasoconstrictor actions of catecholamines and controls the secretion of corticotropin (ACTH), CRH, and vasopressin (ADH) by negative feedback inhibition. Cortisol is vital to the metabolism of carbohydrates and protein, at least in part by stimulating gluconeogenesis and glycogenesis by liver and muscle. It suppresses peripheral cellular uptake and utilization of plasma glucose. It has some control over the immune system by suppressing inflammatory responses and lymphoid tissue. Cortisol stimulates erythrocytosis, maintains normal blood pressure, and counteracts the effects of stress (Oelkers, 1996). Pain, fever, and hypovolemia all result in a sustained increase in the secretion of ACTH and cortisol. During surgical procedures, for example, serum ACTH and cortisol concentrations rise quickly, slowly returning to basal values within 24 to 48 hours. Patients receiving steroid treatment for chronic autoimmune or inflammatory disease need less additional corticosteroid during severe illness and perioperatively than those receiving replacement therapy for adrenal insufficiency (Lamberts et al, 1997).
PHYSIOPATHOLOGY OF SIGNS ATTRIBUTED TO INSUFFICIENT CORTISOL SECRETION
Cortisol Deficiency.
Inadequate glucocorticoid secretion may result from destruction of the adrenal cortex or from dysfunction of the hypothalamus or pituitary gland, with insufficient secretion of CRH or ACTH, respectively. Regardless of the underlying cause, cortisol is necessary physiologically for a number of functions. Lack of cortisol secretion may result in any of a variety of gastrointestinal signs, including anorexia, vomiting, abdominal pain, and weight loss. Energy metabolism is diminished, owing to impaired gluconeogenesis, impaired fat metabolism, decreased fat utilization, and depletion of liver glycogen stores. As a result, fasting hypoglycemia may occur. Associated with these processes could be mental changes, such as diminished vigor and lethargy. Impaired ability to excrete water free of sodium may result in hyponatremia. With primary adrenal insufficiency, there is an unrestrained secretion of ACTH from the pituitary. One of the hallmark signs of hypocortisolism is impaired tolerance to stress, and clinical signs often become more pronounced when the animal is placed in stressful situations.
Iatrogenic Cortisol Deficiency Secondary to Administration of o,p′-DDD.
Dogs with pituitary-dependent hyperadrenocorticism (PDH) are relatively sensitive to o,p′-DDD. These dogs exhibit signs of cortisol deficiency quickly and consistently. Drug-induced complete destruction of the adrenal cortex can follow o,p′-DDD administration, but it is not common if accepted treatment protocols are followed (see Chapter 6). Ketoconazole, an enzyme blocker that interrupts the synthesis of cortisol, can cause severe but reversible cortisol deficiency at doses of approximately 30 mg/kg/day.
Severity of Adrenocortical Glandular Destruction
PATHOGENESIS.
Development of the clinical syndrome associated with adrenocortical insufficiency is believed to require at least 90% destruction of adrenal cortices. Naturally occurring, immune-mediated destruction of the adrenal cortices is usually a gradual process, initially resulting in a “partial deficiency syndrome” characterized by inadequate adrenal reserve, with symptoms manifest only during times of stress (see Table 8-1). Stress may be associated with surgery, trauma, infection, or even psychologic distress, such as when dogs are placed in boarding kennels. However, basal hormone secretion in the unstressed state may be adequate to maintain near-normal plasma electrolyte concentrations and minimal clinical signs. For these dogs, the diagnosis can be confirmed only with tests that assess adrenocortical reserve. As destruction of the adrenal cortices continues, hormone secretion becomes inadequate even under nonstressful conditions, and a true metabolic crisis without any obvious inciting event can result.
TIMING OF EFFECT ON ZONES OF THE ADRENAL CORTEX.
In dogs with primary adrenal insufficiency, each of the three adrenocortical zones seems to become damaged at about the same rate. In other words, aldosterone deficiency (with its associated clinical signs and electrolyte abnormalities) and glucocorticoid deficiency (with its associated clinical signs) occur in tandem. Therefore the ACTH stimulation test (assaying plasma cortisol concentrations) is used to directly assess the capacity for cortisol synthesis and indirectly assess the capacity for aldosterone synthesis. It is extremely rare (but possible) for the zona glomerulosa (aldosterone secretion) to be significantly more or less damaged than the zona fasciculata and zona reticularis (cortisol secretion) (Lobetti, 1998). In contrast, insufficient cortisol response to exogenous ACTH administration in a dog with normal serum electrolyte concentrations could be explained by chronic glucocorticoid administration (common) or pituitary failure to secrete ACTH (rare).
SIGNALMENT
Incidence
The incidence of hypoadrenocorticism (Addison’s disease) suggests the approximate rate at which new cases might be diagnosed. It has been estimated that the average veterinarian in private practice sees about 1400 to 1600 dogs per year and that Addison’s disease occurs in about 0.5 dogs per 1000. Thus the average two-veterinarian practice should expect to diagnose at least one new case yearly (Kelch et al, 1998). The prognosis for treated dogs is excellent, and the average dog should have a normal life span, about 7 years from the average age of diagnosis. The average veterinary practice, therefore, is likely to have a number of addisonian dogs under treatment at any given time.
Dogs Used for Discussion
Hypoadrenocorticism is an uncommon endocrine disorder in dogs, and it is rare in cats. Data have been accumulated from our current series of 205 dogs with naturally occurring hypoadrenocorticism and arbitrarily from 76 and 225 dogs included in two reviews (Willard et al, 1982; Peterson et al, 1996). This collection of 506 dogs with hypoadrenocorticism likely represents a typical group for making generalizations concerning this condition.
Gender
Female dogs account for 349 cases (69%) of the 506 dogs with hypoadrenocorticism. Ninety-one dogs (18%) were castrated males. Predilection for the female is typical for immune-mediated disorders in the dog and may provide crude but further evidence of an immune-mediated pathogenesis for hypoadrenocorticism in most cases (Melian and Peterson, 1996). In a study critically evaluating these parameters, females were about twice as likely to develop Addison’s disease as males. Neutered females and neutered males were each about three times more likely to develop the disease than their intact counterparts (Kelch et al, 1998).
Breed
Significant breed predilections probably exist for this disease. However, virtually every report has documented that mixed-breed dogs are most prevalent and a wide variety of breeds are represented in the case reports and case series that have been published. In our representative dogs, 122 of 506 (24%) were mixed breed. Furthermore, many of the breeds represented reflect those that are simply popular (Table 8-3).
TABLE 8-3 BREED CHARACTERISTICS IN CANINE HYPOADRENOCORTICISM*
| A. Breeds Most Commonly Diagnosed | |
| Mixed breed | 24% |
| Toy or miniature poodle | 10% |
| Labrador Retriever | 9% |
| Rottweilers | 9% |
| Standard Poodle | 8% |
| German Shepherd Dog | 6% |
| Doberman Pinscher | 4% |
| Golden Retriever | 4% |
| West Highland White Terrier | 4% |
| Great Dane | 3% |
| B. Breeds at Increased Risk | |
| Great Dane | |
| West Highland White Terrier | |
| Bearded Collie | |
| Poodle (standard, mini, toy) | |
| Basset hound | |
| C. Breeds at Decreased Risk | |
| Boston Terrier | |
| Dalmatian | |
| Pit Bull Terrier | |
| Boxer | |
| Pomeranian | |
| Yorkshire Terrier | |
| Shetland Sheepdog | |
| Lhasa Apso | |
| D. Breeds That May Have a Genetic Predisposition | |
| Standard Poodle | |
| Portuguese Water Dog | |
| Bearded Collie | |
| Labrador Retriever | |
* From Kelch, W.J. (1996); used with permission
When appropriate statistical analysis is applied to the data, however, several breeds appear to be at increased risk for developing hypoadrenocorticism (see Table 8-3). This implication is based on breeds diagnosed as having Addison’s disease compared with the prevalence of each breed in the overall population. When odds ratios are calculated, the Great Dane, Poodle (Toy, Miniature, and Standard), and West Highland White Terrier are among the breeds with a higher risk. Breeds at lower risk include Lhasa Apsos, Yorkshire Terriers, Boston Terriers, and those of mixed breeding. The Basset Hound, Saint Bernard, and Portuguese Water Dog may be at increased risk, but their lesser popularity, coupled with a small number of cases, precludes a high degree of certainty. Whether the Labrador Retriever is predisposed is not clear. Although reported to be at higher risk in one study, a more rigorous evaluation did not find the breed to be at higher or lower risk (Kelch et al, 1998).
Genetic predispositions for several breeds have been implicated. In one study, hypoadrenocorticism in the Bearded Collie was demonstrated to be highly heritable (Oberbauer et al, 2002). The other breeds that have been claimed to have a genetic predisposition include the Portuguese Water Dog, Standard Poodle, and Labrador Retriever (see Table 8-3). There have also been various reports of canine families afflicted with Addison’s disease. Familial predisposition to hypoadrenocorticism has been suggested in Standard Poodles, Portuguese Water Dogs, Leonbergers, Labrador Retrievers, and other breeds (Auge, 1985; Shaker et al, 1988; Smallwood and Barsanti, 1995). In work underway, a genetic predisposition of Standard Poodles to hypoadrenocorticism, for example, seems to be likely.
HISTORY
Subjectivity of Owner Opinions
The ability to obtain from an owner all information pertinent to providing proper care for the pet is perhaps the most underrated but valuable diagnostic aid in medicine. The veterinarian must realize that some owners, even though quite observant, are not aware of what constitutes a “normal” pet. The best example would be the owners who brought in a severely ill, 2-year-old female Saint Bernard, claiming she had been sick for 2 days. They said that she had been perfectly healthy prior to the present illness. This dog was diagnosed and treated for hypoadrenocorticism. One week after discharge from the hospital, the owners described her as aggressive, playful, filled with energy, possessing an excellent appetite, and more active than she had ever been before. The owners then realized that the dog had been “ill” much of her life but had not demonstrated the type of signs necessary to warrant examination by a veterinarian. This example illustrates the importance to the veterinarian of developing an ability to ask questions and analyze answers without assuming that the owner will mention all relevant information.
Variability in Severity of Clinical Signs
The severity of any given sign can vary dramatically, and “worrisome” problems depend completely on subjective owner opinion. Owner concerns for dogs ultimately diagnosed as having hypoadrenocorticism commonly include poor appetite or anorexia, lethargy or depression, the dog being thin or losing weight, weakness, vomiting and/or regurgitation, diarrhea (sometimes with melena or obvious hematochezia), and/or collapse (Table 8-4). These signs are vague and often suggestive of more common small animal disorders, especially renal, gastrointestinal, and infectious diseases. There are no pathognomonic signs. As previously discussed, any and all signs vary in severity from dog to dog. The suspicion of hypoadrenocorticism is confirmed only when the clinician maintains a differential diagnosis that includes this illness. Correlating the signalment, history, and physical examination findings with a suspicion of adrenal insufficiency allows most practitioners the opportunity to diagnose and treat dogs for this disease.
TABLE 8-4 HISTORICAL OWNER CONCERNS FOR 506 DOGS WITH HYPOADRENOCORTICISM
| Sign | Percentage |
|---|---|
| Poor appetite/anorexia | 88 |
| Lethargy/depression | 85 |
| Thin | 82 |
| Vomiting/regurgitation | 68 |
| Weakness | 51 |
| Weight loss | 40 |
| Diarrhea | 35 |
| Waxing-waning course of illness | 25 |
| Polyuria | 17 |
| Shaking/shivering | 17 |
| Collapse | 10 |
| Painful abdomen | 8 |
Correlating Signs with Hormonal Deficiencies
Each clinical sign described in Table 8-4 can be directly related to a deficiency in glucocorticoid and/or mineralocorticoid secretion. Anorexia, vomiting, regurgitation, lethargy, weakness, loose stools, melena and/or hematochezia, and abdominal pain can be the result of glucocorticoid deficiency alone. These signs, however, are exaggerated if alterations in the plasma sodium and potassium concentrations also exist. Weight loss is a sequela of the problems described, and the “waxing-waning” course is a reflection of progressive but not necessarily absolute deficiency of adrenocortical hormones. Polyuria may be the result of excessive sodium loss into the urine, which causes “washout” of one solute component comprising the renal medullary concentration gradient. The shivering or shaking in dogs with hypoadrenocorticism is believed to be one expression of muscle weakness resulting from depletion of plasma sodium.
PHYSICAL EXAMINATION
Physical examinations completed on the hypoadrenal dogs in our series have not revealed consistent abnormalities, except that the dogs are usually “ill.” Therefore, it should not be surprising that the vague signs of “depression,” lethargy, and appearing thin and weak were the most common abnormalities described among 506 dogs with Addison’s disease (Table 8-5). Dehydration, shock-or-collapse, hypothermia, bradycardia, and weak femoral pulses were detected in only a small number of dogs. Melena and/or hematochezia was occasionally observed after rectal temperature assessment or on rectal examination. Abdominal pain, hypothermia, and emaciation have been mentioned in the veterinary literature but have only rarely been seen by us or in the reports used in this series (Willard et al, 1982; Peterson et al, 1996).
TABLE 8-5 ABNORMALITIES NOTED ON PHYSICAL EXAMINATION OF 506 DOGS WITH HYPOADRENOCORTICISM
| Sign | Percentage |
|---|---|
| Depression/lethargy | 87 |
| Thinness | 82 |
| Weakness | 66 |
| Dehydration | 41 |
| Shock/collapse | 24 |
| Bradycardia | 22 |
| Weak femoral pulse | 22 |
| Melena/hematochezia | 17 |
| Hypothermia | 15 |
| Abdominal pain | 7 |
CLINICAL PATHOLOGY
Erythrocyte Parameters
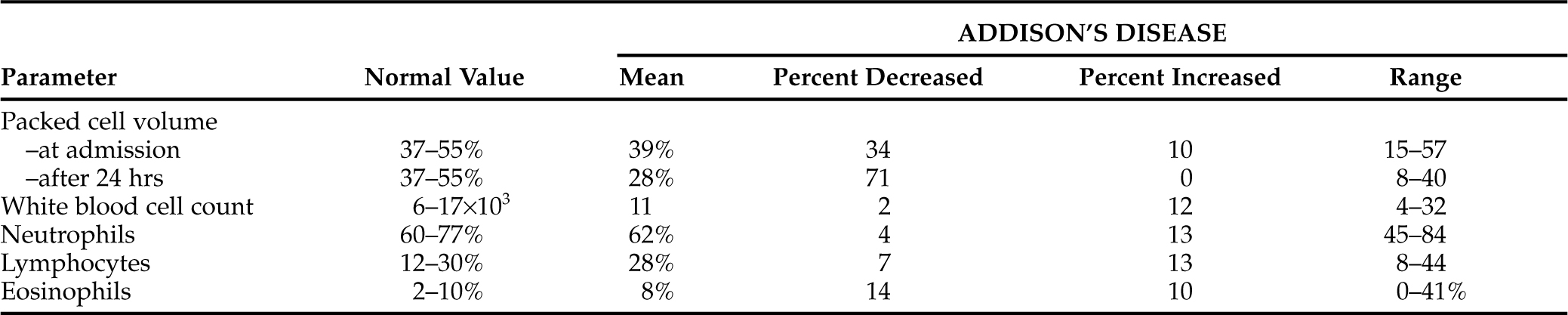
In adrenal insufficiency, a normocytic normochromic anemia accompanied by little or no reticulocyte response secondary to bone marrow suppression from hypocortisolism is common. However, if an animal becomes hemoconcentrated secondary to dehydration, the underlying anemia may not be obvious on the initial complete blood count (CBC) (Table 8-6). Once rehydrated, these dogs usually exhibit the typical mild anemia of hypoadrenocorticism, with hematocrits of 20% to 35% being typical.
If the anemia at the time of initial physical examination is significant and the dog is in an “addisonian” crisis, rehydration may reduce the circulating red blood cell count to life-threatening levels. Severe anemia is usually associated with chronic bone marrow suppression and/or acute and significant gastrointestinal hemorrhage. Gastrointestinal hemorrhage can be specifically caused by glucocorticoid deficiency. Transfusion of red cells (fresh whole blood or packed cells) in these situations may be a critical component of patient management (Medinger et al, 1993).
Leukocyte Parameters
TOTAL WHITE BLOOD CELL (WBC) COUNTS.
Total WBC counts in hypoadrenal dogs vary from low normal to mildly increased. Most of these dogs have normal total WBC counts. No consistent WBC differential count was obvious in our series of dogs. An increase in the WBC count may reflect a granulocytic response to concurrent bacterial infection (see Table 8-6).
EOSINOPHILS.
The presence of a normal absolute eosinophil count in an ill dog may be significant because a stress pattern with no or few eosinophils is expected in such dogs if they have normal adrenocortical function. The finding of a normal eosinophil count in a stressed or ill dog should be viewed with a suspicion for hypoadrenocorticism. It must be remembered that a relative or absolute eosinophilia may also occur with many other diseases and, when present, eosinophilia can be used in developing a differential diagnosis that may lead to an explanation or cause for the illness (Table 8-7) (Rothenberg, 1998). The presence or absence of eosinophils should be considered a nonspecific and insensitive means of deciding the likelihood that any animal may or may not have hypoadrenocorticism.
TABLE 8-7 POTENTIAL CAUSES OF EOSINOPHILIA IN DOGS AND CATS
LYMPHOCYTES.
Animals with normal adrenal function respond to stress in part by secreting cortisol. Glucocorticoids decrease lymphocyte numbers in the circulation. Therefore ill and untreated hypoadrenal dogs should have a relative or absolute lymphocytosis. However, as with eosinophils, lymphocyte numbers or percentages have not provided consistent, sensitive, or specific alterations that can be considered classic for hypoadrenocorticism (see Table 8-6). This parameter could be used as a “clue” to adrenocortical health. Hypoadrenocorticism should be suspected when normal or increased lymphocyte counts are present in an ill dog. Thirteen percent of our 506 hypoadrenal dogs had lymphocytosis, and 80% had normal absolute counts.
MODIFIED THORN TEST.
Before assays were readily available to easily measure plasma cortisol concentrations, indirect tests of adrenocortical function were used. Included were tests that assessed changes in WBC numbers and/or percentages of certain WBCs present in the circulation after an injection of ACTH. A “modified Thorn test” (named after Dr. G. W. Thorn, who described such a protocol in 1948) has been reviewed. The recommendation for dogs was to obtain blood samples before and 4 hours after ACTH administration. Dogs with normal adrenocortical function demonstrate an increase in the neutrophil: lymphocyte ratio of at least 30%, and eosinophils decrease by at least 50% from pre-ACTH values (Chastain et al, 1989). This test was recommended as simple and readily available for determining the need for plasma cortisol measurements. We do not use this test.
Serum Electrolytes: “Classic” Hyponatremia and Hyperkalemia
PATHOPHYSIOLOGY OF SERUM ELECTROLYTE ALTERATIONS.
The classic electrolyte alterations in Addison’s disease are hyponatremia, hypochloremia, and hyperkalemia. These abnormalities are due primarily to aldosterone deficiency causing failure of the kidneys to conserve sodium or to excrete potassium. Hyponatremia is primarily caused by renal sodium wasting. Sodium lost via the kidneys is accompanied by water, resulting in both hyponatremia and dehydration should fluid intake not compensate for urinary losses. To some extent this dehydration may mask sodium depletion (mild or severe). Deficiency in adrenocortical hormones allows greater amounts of sodium to pass into the intracellular compartment as intracellular potassium concentrations decrease. Hyperkalemia results from both a shift of potassium from intracellular to extracellular compartments and from a decrease in renal excretion. The former condition results from a loss of cortisol effects on the sodium-potassium pump, which normally maintains a gradient across cellular membranes (Nelson, 1980).
Hypoaldosteronism and acidosis enhance the shift of potassium from intracellular to extracellular compartments. Decreased potassium exchange for sodium in the distal renal tubule leads to decreased urinary potassium excretion and increased sodium excretion. The shift in electrolytes between body compartments may be partly corrected by the administration of cortisol, but aldosterone or another mineralocorticoid is necessary to prevent renal loss of sodium and retention of potassium (Tyrrell et al, 1991).
SODIUM.
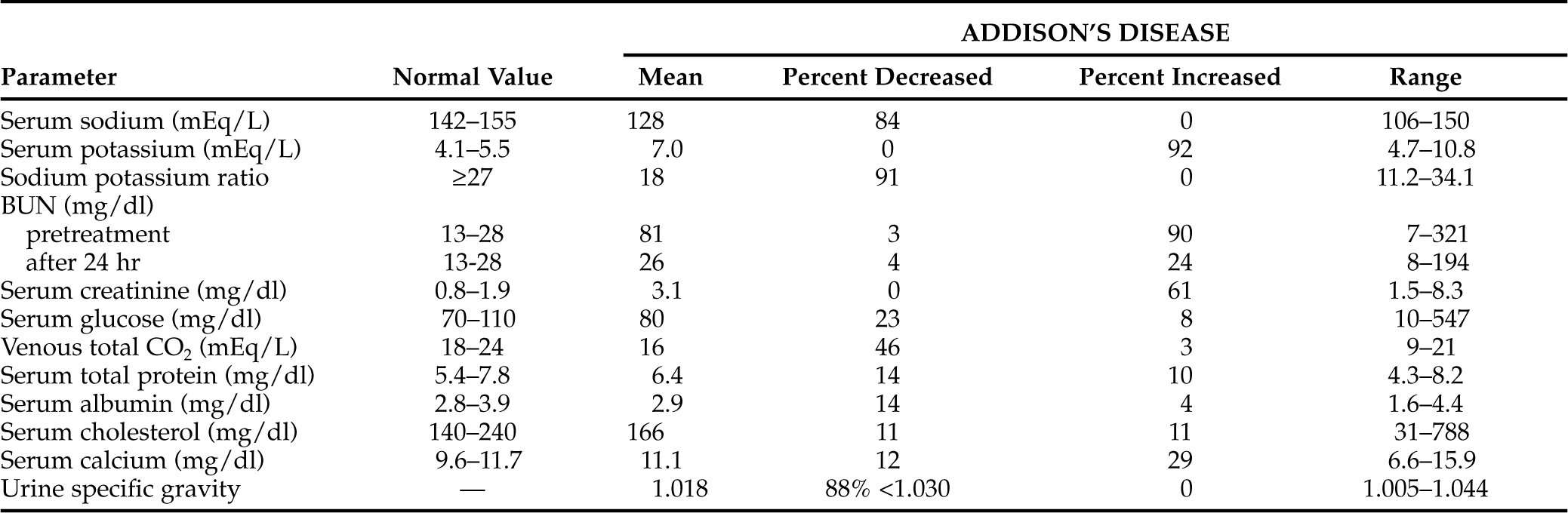
Serum sodium concentrations have varied from normal to as low as 106 mEq/L at the time of diagnosis of Addison’s disease in dogs (Table 8-8). Of 483 dogs with primary hypoadrenocorticism, 417 (86%) had serum sodium concentrations less than 142 mEq/L at the time of diagnosis. In addition, eight of the 23 dogs with apparent ACTH deficiency (secondary adrenocortical deficiency) were hyponatremic at the time of diagnosis. In 60 hypoadrenal dogs described in a separate study, the mean serum sodium concentration at the time of diagnosis was 128 mEq/L (Lynn et al, 1993), a value similar to the mean value for the 506 dogs.
POTASSIUM.
Serum potassium concentrations in dogs at the time hypoadrenocorticism is diagnosed vary from normal to extremely increased levels that induce clinically obvious cardiac rhythm disturbances (see Table 8-8). Of the 483 hypoadrenal dogs in this review, 460 (95%) had serum potassium concentrations greater than 5.5 mEq/L at the time of diagnosis. In 60 of our more recently diagnosed hypoadrenal dogs, the mean serum potassium concentration was 7.2 mEq/L, and in the 506 dogs it was 7.0 mEq/L. None of the 23 dogs with apparent ACTH deficiency had hyperkalemia.
SODIUM: POTASSIUM RATIO: VALUE AND LIMITATIONS.
The sodium to potassium ratio has frequently been used as a diagnostic tool to aid in gaining a suspicion or in specific identification of dogs with adrenal insufficiency. The normal ratio varies between 27:1 and 40:1. Values are often below 27:1 and may be below 20:1 in dogs with primary hypoadrenocorticism. Determination of serum electrolyte concentrations from dogs suspected of having adrenal insufficiency is of paramount importance. The finding of the classic electrolyte abnormalities, or of hyperkalemia without a decrease in the serum sodium concentration or vice versa (decreases in serum sodium without an increase in the serum potassium concentration) should prompt immediate therapy. The assumption that the clinician may be treating Addison’s disease is warranted and may be lifesaving.
If the provisional diagnosis of hypoadrenocorticism made on the basis of serum electrolyte concentrations is incorrect, emergency therapy is rarely harmful. Aggressive management of hypoadrenocorticism is not significantly different from that for life-threatening renal or gastrointestinal diseases. However, the limitations of a diagnosis based solely on serum electrolyte determinations must be realized. Reliance on serum electrolyte concentrations as the sole criterion for diagnosing adrenal insufficiency can be misleading if three factors are not kept in mind (see next three sections): first and most important is the slow, progressive nature of the development of primary adrenal insufficiency in many dogs; second, dogs with pituitary failure continue to secrete aldosterone; and third, hyperkalemia and hyponatremia are not pathognomonic for adrenal insufficiency (Figs. 8-4 and 8-5).
SERUM ELECTROLYTE ASSESSMENT IS NOT ALWAYS DEFINITIVE
Insidious Illness.
Only 17 of the 483 dogs (4%) with primary adrenocortical failure from our series and the two additional studies used had normal serum electrolyte concentrations at the time of diagnosis (see Table 8-8). Twelve of those 17 dogs developed typical electrolyte abnormalities weeks to months after glucocorticoid therapy (without mineralocorticoid support) was initiated. This uncommon finding of normal serum electrolyte concentrations has also been recognized by other investigators (Rogers et al, 1981; Bartges and Nielson, 1992; Schaer, 1994; Peterson et al, 1996). This does not include dogs with secondary adrenocortical atrophy due to pituitary ACTH deficiency. The diagnosis of Addison’s disease in dogs with normal serum electrolyte parameters may be more obvious once the results of an ACTH stimulation test are available. The challenge rests in maintaining a clinical suspicion for hypoadrenocorticism and deciding to per-form an ACTH stimulation test when the serum electrolyte concentrations are not suggestive of the diagnosis.
Pituitary ACTH Deficiency (Secondary Adrenocortical Failure).
Volume depletion, dehydration, and serum potassium abnormalities are usually absent because aldosterone is only minimally affected by ACTH. Hypotension is usually not present except in acute presentations. Hyponatremia was documented in 8 of 23 dogs in the series included here, and it may have been the result of water retention, anorexia, and vomiting and/or diarrhea. Inability to excrete a water load is not typically accompanied by hyperkalemia. Prominent clinical features are weakness, lethargy, anorexia, and occasionally vomiting. Joint, muscle, and/or abdominal pain may be apparent. Hypoglycemia is occasionally the presenting feature (Table 8-9). Acute decompensation and shock may occur.
TABLE 8-9 SELECTED SERUM BIOCHEMISTRY VALUES IN 23 DOGS WITH NATURALLY OCCURRING SECONDARY HYPOADRENOCORTICISM
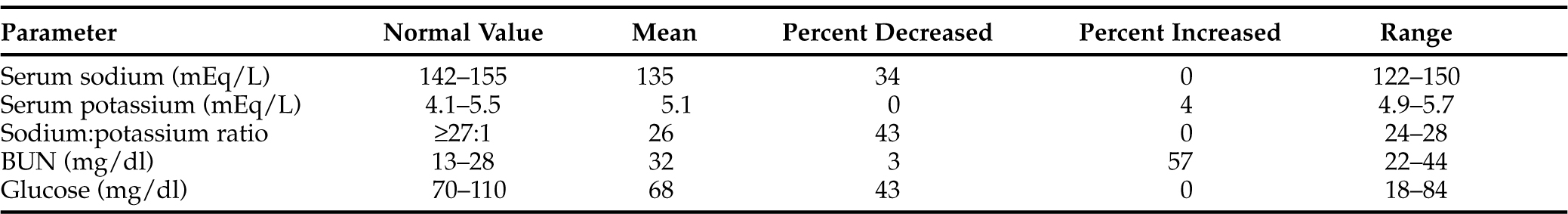
ACTH deficiency may occur as a result of a primary pituitary problem (trauma, infection, cancer) or secondary to long-term corticosteroid medication that is acutely discontinued (see Fig. 8-1). Clinically, these dogs may be indistinguishable from dogs with primary adrenal insufficiency or those with renal or gastrointestinal problems. Only a thorough medical history can alert the veterinarian to the possibility of secondary adrenal insufficiency.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree