Chapter 7 HYPERADRENOCORTICISM IN CATS (CUSHING’S SYNDROME)
In 1932, Dr. Harvey Cushing authored a report in which he described a group of people with a disorder that he suggested was “the result of pituitary basophilism.” Subsequent study of the clinical, biochemical, and histologic features of these individuals indicate that each had been afflicted with a syndrome resulting from chronic exposure to excesses in circulating cortisol concentrations. The eponym Cushing’s syndrome is an “umbrella” term referring to this condition (hyperadrenocorticism) in people and, more recently, in animals. Chronic cortisol excess may occur secondary to iatrogenic cortisol administration, or it may occur with naturally occurring disease. Thus a pathophysiologic classification of causes for Cushing’s syndrome includes several different conditions. In addition to the iatrogenic condition, the naturally occurring disorder can be caused by a pituitary tumor secreting excess ACTH with subsequent adrenocortical hyperplasia and excess adrenocortical cortisol secretion. The other common naturally occurring condition in animals is that caused by an autonomously functioning, cortisol-secreting, adrenocortical tumor. As discussed later, adrenocortical tumors possess the ability to synthesize steroid hormones other than cortisol. Such cortisol-secreting tumors have been documented in cats together with two additional syndromes (progesterone and aldosterone excess).
Veterinary medicine, especially canine and feline veterinary medicine, has received support from studies describing conditions, diagnoses, and management of diseases acquired by human beings. Thus studies by physicians have been of great benefit to veterinarians. Human beings are the animal model most frequently studied by veterinarians. In many situations, what has been learned about people is then applied to dogs. Often, what has been learned and applied to dogs is then applied to cats. In some situations, illnesses in cats may be analogous to conditions in dogs, whereas other illnesses may be analogous to the conditions in people. Some feline diseases, of course, are unique. With respect to hyperadrenocorticism (Cushing’s syndrome), there are more similarities when comparing cats with people than there are when comparing cats with dogs. One of the more obvious comparisons involves the incidence of hyperadrenocorticism. In dogs this endocrine disorder is surprisingly common, whereas in people and cats it is quite uncommon.
We assume that the underlying physiologic causes for hyperadrenocorticism are similar in people, dogs, and cats. Thus the reader interested in applied physiology and in mechanisms of disease is encouraged to review the appropriate sections in Chapter 6. The focus of this chapter is a review of the clinical condition of hyperadrenocorticism in cats. To aid in this discussion, information from the records of 46 cats diagnosed as having naturally occurring hyperadrenocorticism at our hospital were added to the information from an additional 24 cats described in the literature (Immink et al, 1992; Daley et al, 1993; Goossens et al, 1995; Watson and Herrtage, 1998; Moore et al, 2000a; Meij et al, 2001), for a total of 70 cats with hyperadrenocorticism. This literature includes only those reports published since 1992. We chose reports from this time period in order to be sure that valid and currently available assays were used in the assessment of each cat and that current concepts in diagnosis and treatment were used. Before 1990, only a few cats with this disease were mentioned in the literature (Meijer et al, 1978; Peterson and Steele, 1986; Zerbe et al, 1987; Nelson et al, 1998). Furthermore, in the reports we arbitrarily used, we set the following selection criteria: each cat must have had some clinical signs associated with Cushing’s syndrome, each diagnosis must have been confirmed with appropriate screening test results, and each cat must have had advanced imaging (computed tomography [CT] or magnetic resonance imaging [MRI] scans) confirmation or histologic confirmation of the diagnosis. Reports that did not meet these inclusion criteria were not used.
ETIOLOGY
Iatrogenic Cushing’s Syndrome
Several studies on laboratory cats experimentally treated with glucocorticoids have been published. Those treated for a 4-week period had few abnormalities on physical examination and no consistent hematologic or biochemical changes (Scott et al, 1979; Scott et al, 1982). When treated for 9 weeks or longer, some cats exhibited polydipsia, polyuria, polyphagia, abdominal enlargement, thin skin, and curling of their ear tips. Some of these cats also developed liver enlargement, muscle wasting, ecchymoses, and skin fragility (Scott et al, 1982). They also tended to develop mild hyperglycemia, mild hypercholesterolemia, glycogen accumulation in hepatocytes, and a vacuolar hepatopathy. Cataracts developed in some laboratory cats treated with topical glucocorticoids (Brightman, 1982; Zhan et al, 1992). The muscle weakness associated with glucocorticoid administration tends to be most pronounced in the muscles with fast twitch fibers (Robinson and Clamann, 1988).
Three privately owned cats have been described that had iatrogenic chronic glucocorticoid excesses. The cats had received steroids for stomatitis, feline infectious peritonitis (FIP), and pruritus (Green et al, 1995; Schaer and Ginn, 1999; Ferasin, 2001). At the time of final presentation, one of the three cats was extremely ill as a result of FIP. Among the common features noted in these cats after chronic glucocorticoid exposure were abdominal enlargement, muscle wasting, poor hair coats, and skin fragility. The skin fragility included easy bruisability, skin tears, and thin skin. Two cats had increases in liver enzymes and hepatic vacuolar hepatopathy thought to be similar to those seen in dogs. The most important information derived from these three reports is the realization that iatrogenic Cushing’s syndrome in cats is uncommon. In addition, some features seen in cats chronically treated with exogenous steroids are not seen in cats with naturally occurring Cushing’s syndrome.
Naturally Occurring Feline Cushing’s Syndrome (FCS)
The causes of naturally occurring feline Cushing’s syndrome (FCS) are similar to those recognized in human beings and dogs. It has been suggested that resistance to glucocorticoid-induced side effects, likely to exist in cats, may help explain the relative low rate of naturally occurring disease diagnosis as compared with the incidence of the naturally occurring syndrome in dogs. In other words, if excess glucocorticoids cause few clinical signs in cats, how would the diagnosis ever be suspected in the first place? However, the incidence of the naturally occurring disease in cats (assessed nonscientifically) appears to be similar to the incidence of the syndrome in human beings; yet people are sensitive to the effects of glucocorticoids. Therefore it is our suspicion that naturally occurring FCS is simply less common among cats as compared with dogs.
SIGNALMENT (AGE, SEX, BREED)
Hyperadrenocorticism is a disease of middle-aged and older cats. As can be seen in Table 7-1, the mean age of 39 cats diagnosed as having PDH was 10.7 years with a range of 5 to 16 years. The mean age among 12 cats with a functioning adrenocortical tumor was 12 years, with a range of 8 to 15 years. The mean age for all 51 cats was just under 11 years. Among these same 51 cats, there were 25 males and 26 females. Almost all cats that have been diagnosed as having naturally occurring FCS have been neutered. A variety of breeds are included among the cats diagnosed with hyperadrenocorticism. As seen in Table 7-2, the most commonly afflicted breed is the domestic short-haired cat (31 of 51 cats; 61%), and if domestic long-haired cats are added to that group, 38 of the 51 cats (75%) are represented. Cats representing various other breeds have been diagnosed with FCS.
TABLE 7-1 AGE AT TIME OF HYPERADRENOCORTICISM DIAGNOSIS IN CATS (51 CATS)*
| Age (Years) | Pituitary Dependent Number of Cats | Adrenocortical Tumor Number of Cats |
|---|---|---|
| 5 | 1 | – |
| 6 | 5 | – |
| 7 | 2 | – |
| 8 | 3 | 1 |
| 9 | 3 | 2 |
| 10 | 4 | – |
| 11 | 4 | 1 |
| 12 | 5 | 3 |
| 13 | 3 | 1 |
| 14 | 4 | 2 |
| 15 | 2 | 2 |
| 16 | 3 | – |
| Mean | 10.7 years | 12 years |
* Mean age of all 51 cats was 10.9 years
DURATION OF CLINICAL SIGNS, CHIEF COMPLAINT, AND GENERAL HISTORY
Duration of Clinical Signs
In our review of records from cats diagnosed with FCS, the duration of clinical signs or the duration of specific owner concerns were available for 43 cats (Table 7-3). The range in duration of signs was from as little as 1 month to greater than 12 months. Forty-one of the 43 cats with either PDH or ATH had clinical signs for less than a year.
TABLE 7-3 DURATION OF CLINICAL SIGNS PRECEDING DIAGNOSIS OF HYPERADRENOCORTICISM IN CATS (43 CATS)
| Duration (Months) | Number of Cats with Pituitary-Dependent Disease | Number of Cats with Adrenocortical Tumor |
|---|---|---|
| 1 | 2 | – |
| 2 | 5 | 1 |
| 3 | 5 | 1 |
| 4 | 3 | 2 |
| 5 | 3 | 2 |
| 6 | 3 | 1 |
| 7 | 1 | 3 |
| 8 | 2 | – |
| 9 | 1 | – |
| 10 | 2 | – |
| 11 | – | – |
| 12 | 2 | 2 |
| >12 | 2 | – |
Owner Chief Complaints
The chief complaints noted in records from 48 cats with FCS included “difficult to regulate diabetes mellitus” in 26 cats (54%). Most commonly, diabetes mellitus was classified as difficult to regulate when owners felt that their pet had persistence of polyuria, polydipsia, and/or polyphagia despite administration of insulin (Table 7-4). Less frequently, owners believed their cats’ diabetes mellitus was poorly controlled if their pet lost weight, failed to gain weight, remained lethargic, or had poor grooming habits. It was not always obvious from the records whether or not an owner believed that any of these problems was directly due to inadequate control of diabetes mellitus. Often, insulin dose and type had been altered numerous times by the primary care veterinarian in an effort to gain control of the diabetes mellitus prior to referral.
TABLE 7-4 CHIEF COMPLAINTS BY OWNERS OF CATS ULTIMATELY DIAGNOSED AS HAVING HYPERADRENOCORTICISM (48 CATS – TOTAL; 37 OF THESE 48 CATS HAD DIABETES MELLITUS AND 11 DID NOT HAVE DIABETES MELLITUS)
| Complaint | Pituitary Dependent (36 Cats) | Adrenocortical Tumor (12 Cats) |
|---|---|---|
| Resistant diabetes mellitus (PD/PU/PP)* | 22 | 4 |
| 6 | 2 | |
| Fragile (torn) skin | 3 | 3 |
| Weight loss | 2 | – |
| Lethargy | 2 | 2 |
| Alopecia/failure to regrow hair | 2 | 2 |
| Diarrhea | 2 | – |
| Weakness | 1 | – |
| Vomiting | 1 | – |
| Abdominal enlargement | 1 | 1 |
| Not grooming | 1 | – |
* PD, polydipsia; PU, polyuria; PP, polyphagia.
Of the 48 cats in this evaluation, 37 had diabetes mellitus. Eleven of the 37 diabetic cats were believed to be well controlled with insulin. Of the 11 nondiabetic cats, 8 were believed to have polyuria and polydipsia (PU/PD); this problem was established as the owners’ chief complaint. Other chief complaints varied and were relatively less common (see Table 7-4). Among the uncommon primary owner concerns were fragile (torn) skin in six cats (12%), lethargy in four cats, and alopecia or failure to regrow hair that had been previously shaved in four cats.
Owner Observations
SUMMARY OF OBSERVATIONS.
Owner observations were available from 62 cats diagnosed as having FCS (Table 7-5). The most common concerns noted in the histories obtained from these owners was PU/PD in 51 cats (82%), polyphagia in 42 cats (68%), weight loss or failure to gain weight in 32 cats (52%), and lethargy or reports of “sleeps more” in 29 cats (47%). Concerns about skin problems were noted 61 times. These concerns ranged from the extremely worrisome fragile or torn skin noted in six cats, to a failure to grow hair after it had been shaved (usually for venipuncture or abdominal ultrasonography examination). There were also concerns over grooming excessively or not enough and concerns about a hair coat that had become unusually coarse. Multiple skin problems were noted in some cats, whereas others had none. The owners of 21 cats with PDH (from the total of 50, or 42%) and the owners of 3 cats with ATH (from the total of 12, or 25%) did not observe any problem relative to the skin or hair coat. Similar owner observations in cats with PDH as compared with those with ATH underscores the final common denominator of disease in these cats, that is, chronic exposure to excesses in circulating cortisol.
TABLE 7-5 OWNER OBSERVATIONS IN CATS WITH NATURALLY OCCURRING HYPERADRENOCORTICISM (TOTAL OF 62 CATS)
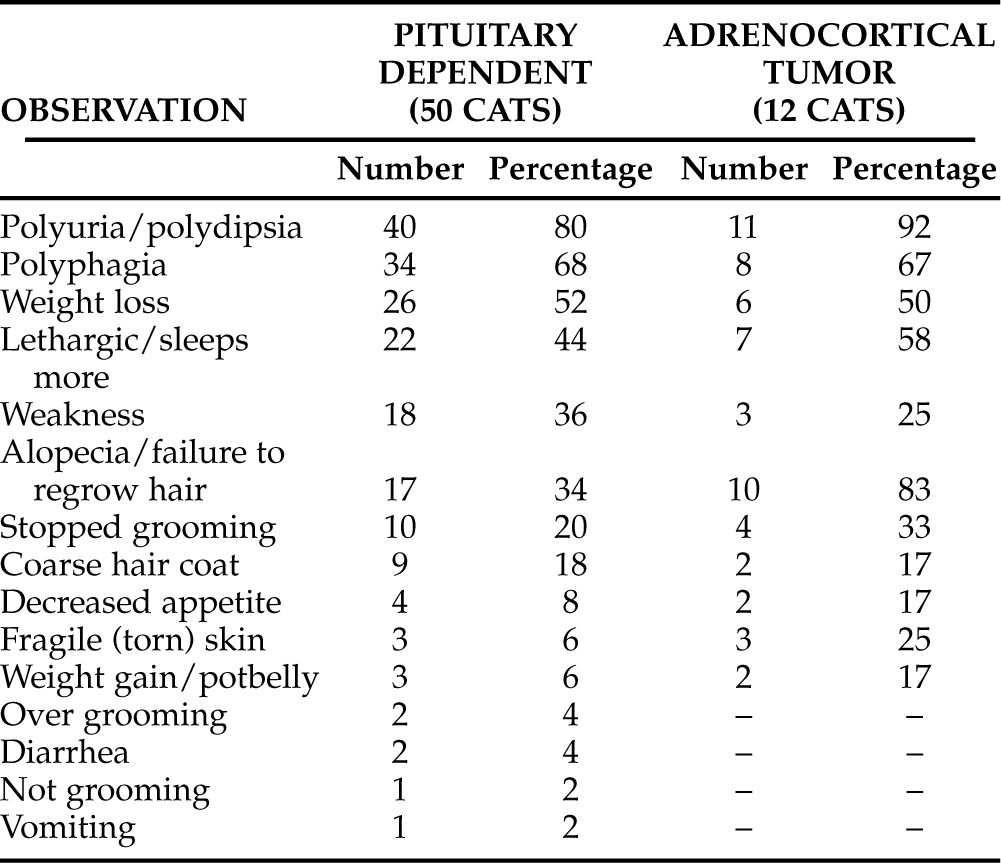
It has been suggested that clinical signs of hyperadrenocorticism are not commonly detected by owners or veterinarians until cats with FCS develop diabetes mellitus. The results of this review suggest that this is true for a majority of afflicted cats. However, some of the signs in these cats did precede development of diabetes mellitus, and other cats never developed diabetes. Thus a suspicion of FCS may result from a history and physical examination in nondiabetic cats. Such abnormalities as thin skin, skin fragility, “potbelly” appearance, muscle atrophy, weakness, PU/PD, and various hair coat disorders might lead to the diagnosis of FCS in nondiabetic cats.
PHYSICAL EXAMINATION ABNORMALITIES
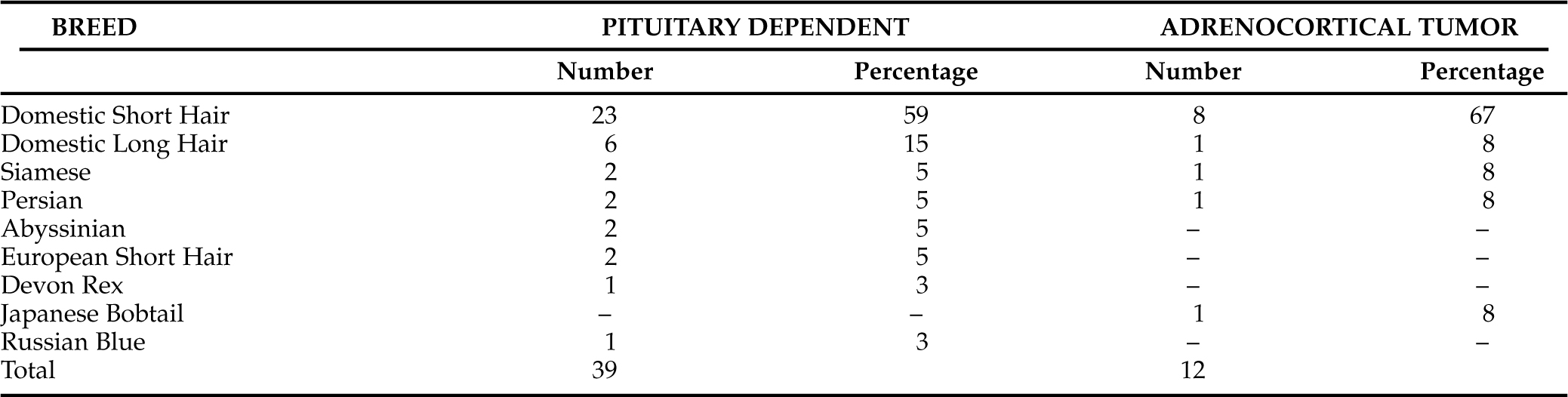
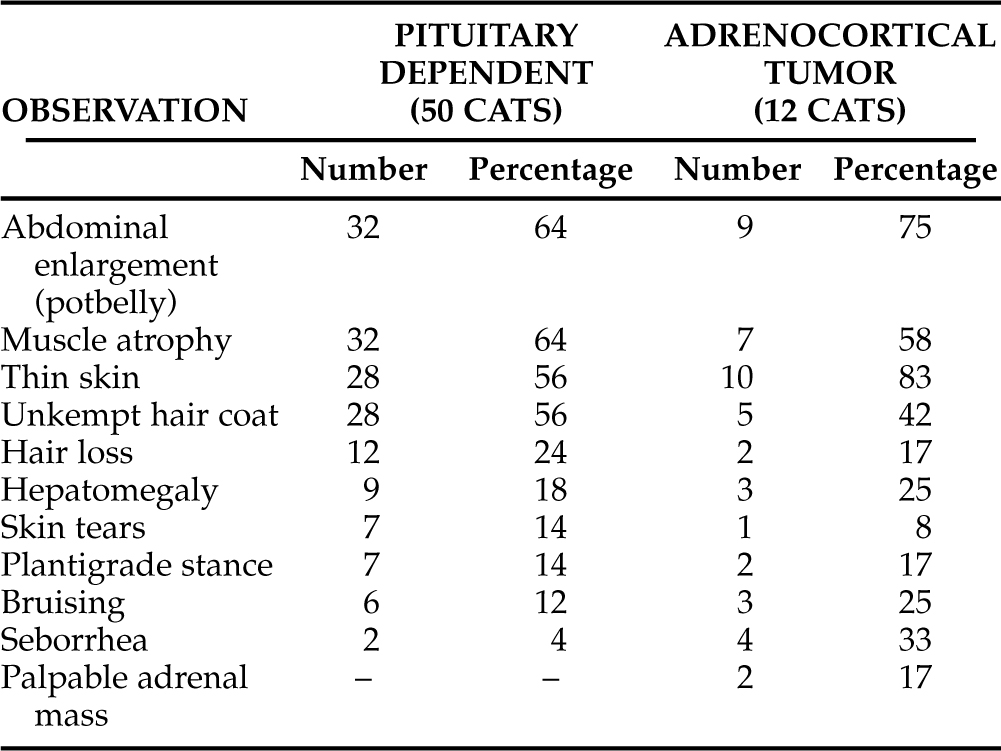
The physical examination abnormalities from 62 cats with naturally occurring hyperadrenocorticism are listed in Table 7-6. Observations from cats with PDH are separated from cats with ATH. As can be appreciated, many of the previously described owner concerns were obvious to the veterinarian performing the physical examination. The most common abnormalities include obvious abdominal enlargement detected in 41 cats (66%; Fig. 7-1, A and B), muscle atrophy detected in 39 cats (63%), thin skin detected in 38 cats (61%), and an “unkempt” hair coat detected in 33 cats (53%). Less common abnormalities include hair loss, hepatomegaly, skin tears, plantigrade stance of the rear legs, bruising, and seborrhea. In one report, each of two cats with an adrenocortical tumor had palpable abdominal masses that were subsequently demonstrated to be neoplastic adrenal glands (Immink et al, 1992). The finding of a palpable adrenal tumor is considered quite unusual.
TABLE 7-6 PHYSICAL EXAMINATION ABNORMALITIES IN CATS WITH NATURALLY OCCURRING HYPERADRENOCORTICISM (TOTAL OF 62 CATS)
EXPLANATIONS FOR THE HISTORY AND PHYSICAL EXAMINATION ABNORMALITIES
Weakness, Lethargy, Pot Belly, and Bruising
Explanations for these clinical signs can be directly related to the physiologic effects of cortisol. Cortisol is protein-catabolic and therefore causes the breakdown of muscle. Muscle wasting results in weakness, which may be obvious to owners. Alternatively, weakness may appear to an owner to be lethargy or an increase in the amount of time spent sleeping. Furthermore, some cats with FCS have a plantigrade posture, which is often related to the neuropathy associated with diabetes mellitus in cats. Chronic cortisol excess is also well recognized to cause a redistribution of fat from areas throughout the body with specific increases in abdominal (mesenteric) fat deposition. This classic abnormality is common in human beings, dogs, and cats. The increase in abdominal fat content coupled with muscle wasting results in the pot belly appearance that is classic of Cushing’s syndrome in all species. A pot belly is the clinical consequence of increased weight of abdominal content pressing down upon weakened abdominal musculature (see Fig. 7-1, A and B).
Cortisol also causes a relative decrease in the ability to heal because of blood vessel friability and a decrease in fibrous response to injury. Loss of subcutaneous fat (mobilized to the abdomen), friability of blood vessels that are more superficial because of loss of fat protection, and decreases in normal healing properties increase the predisposition to bruising in these cats. In many cats, bruising after venipuncture or clipping of hair can be dramatic.
Dermatologic Abnormalities
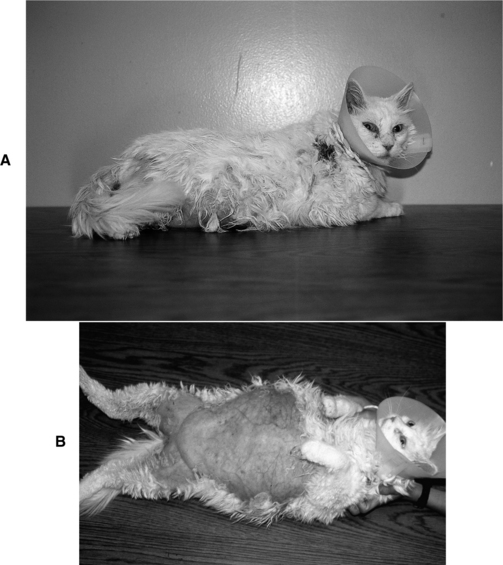
Bilateral symmetric nonpruritic alopecia is a classic and common feature of Cushing’s syndrome in dogs but not a typical abnormality in cats. The alopecia that most FCS cat owners observe is due to a failure to regrow hair that has been shaved by veterinarians or hair lost as a result of normal or excessive grooming (Fig. 7-2, A and B). It is difficult to know whether “excessive” grooming is truly an abnormality. Are these cats grooming normally but causing hair loss, thereby also causing owner concern? Or are they licking themselves more than normal? The failure to regrow lost hair is most likely secondary to atrophy of the hair follicles. This atrophy disrupts the hair shaft attachment to the follicle. Any new hair that does grow tends to be brittle, sparse, and fine.
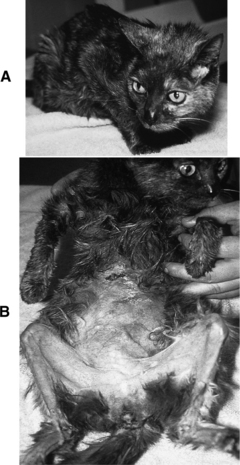
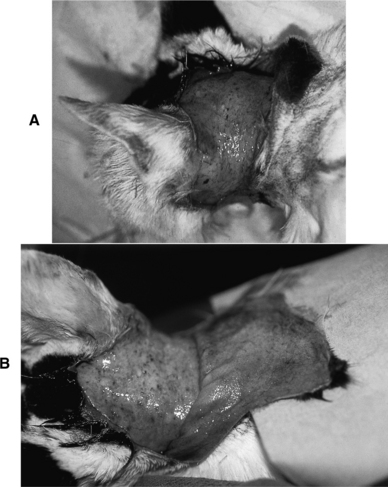
Thin skin (Fig. 7-3, A and B), poor wound healing, and susceptibility to infection are typical sequelae to chronic excesses in circulating cortisol. Suppression of the immune system in individuals with Cushing’s syndrome exaggerates these problems. Skin fragility is not typical of canine Cushing’s syndrome but is well recognized in afflicted people and is well described but not common in cats (Fig. 7-4, A and B; see Tables 7-5 and 7-6). These dermatologic abnormalities are serious and can result in life-threatening overwhelming sepsis. Thin skin is an expression of the catabolic effects of cortisol.
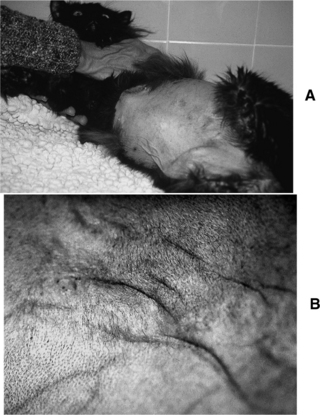
FIGURE 7-3 A, 11-year-old male cat with feline Cushing’s syndrome (FCS). This cat had a progesterone-secreting tumor (see page 391). B, Note the thin skin sometimes associated with chronic exposure to excess cortisol.
ROUTINE CLINICAL PATHOLOGY (CBC, SERUM BIOCHEMISTRY, URINALYSIS)
Complete Blood Count (CBC)
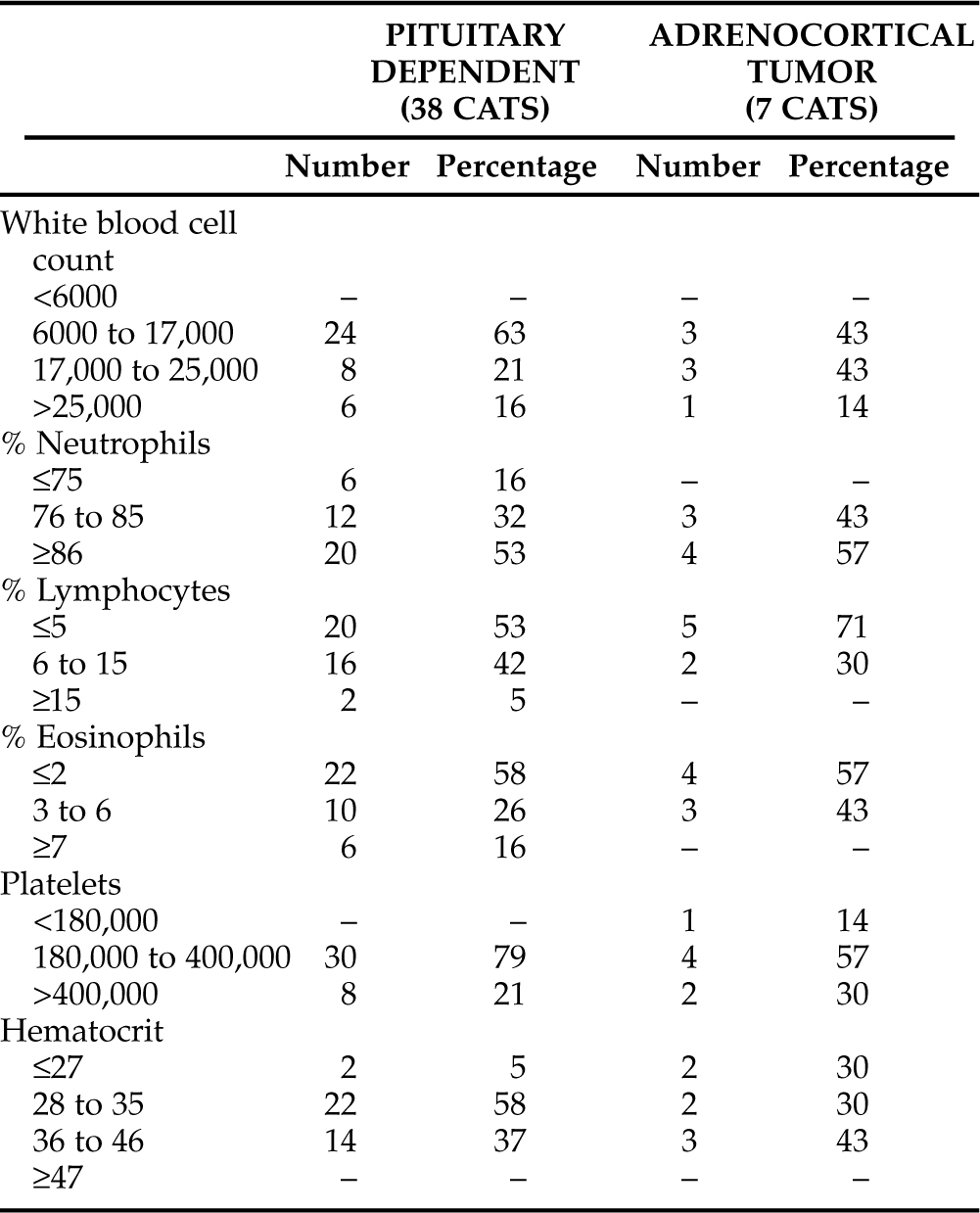
Complete blood count (CBC) results from 45 cats with naturally occurring hyperadrenocorticism can be found in Table 7-7. The most important feature of these results is the lack of consistent abnormalities. That is, none of the results seem “classic.” A majority of afflicted cats had a “stress leukogram” (neutrophilia and reduction in lymphocyte and eosinophil percentages). Of the 45 cats, 24 (53%) had a neutrophil percentage of >86%, 25 (56%) had a lymphocyte percentage of <5%, and 26 (58%) had an eosinophil percentage of <2% of the total white cell count. As can be seen, therefore, stress leukograms were identified, but this was not a consistent feature of the CBC results. No cat was leukopenic, one was thrombocytopenic, and four were anemic. Of the four anemic cats, only one had a hematocrit below 24% (that result was 16%).
Serum Biochemistry and Urinalysis
LIVER ENZYMES.
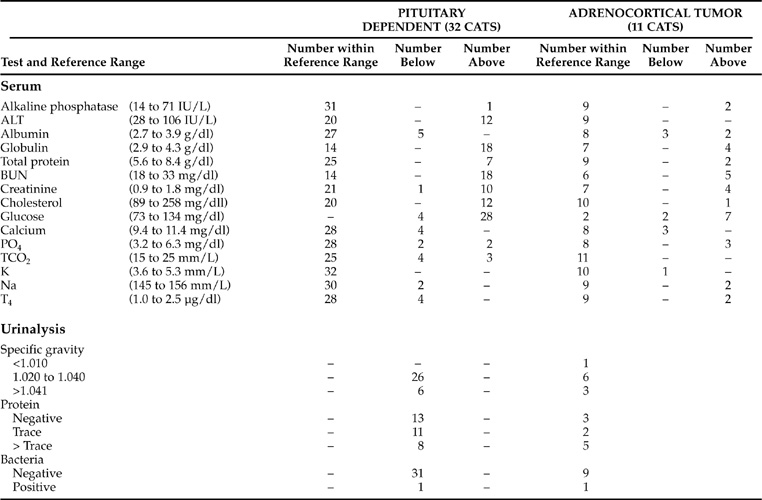
The introduction to this section discusses the common routine biochemical test result abnormalities from dogs with naturally occurring or iatrogenic Cushing’s syndrome. The results from cats with this endocrine disorder are strikingly different (Table 7-8). Although dramatic increases in serum alkaline phosphatase (SAP) activities (average >1,000 IU/L) are the most common biochemical abnormality in dogs, only 3 of 43 cats (7%) with naturally occurring Cushing’s syndrome had an increase in serum alkaline phosphatase activity. Another common abnormality in dogs with Cushing’s syndrome is mild to moderate increase in serum alanine aminotransferase (ALT) activity. This abnormality is identified in more than 50% of dogs, but occurred in only 12 of 43 cats (28%). Several of the cats with increases in ALT activity had significant hepatopathies that were not related to their Cushing’s syndrome. Dogs with iatrogenic or naturally occurring excesses in serum cortisol concentration commonly develop “steroid hepatopathy,” as well as significant circulating concentrations of the “steroid-induced” isoenzyme of SAP. Neither of these typical canine features of Cushing’s syndrome is believed to occur in cats or human beings. However, “steroid hepatopathy” that included liver enlargement and a vacuolar appearance to hepatocytes has been occasionally described in cats with FCS.
SERUM CHOLESTEROL AND T4.
Increases in serum cholesterol concentration are identified in 60% to 70% of dogs with Cushing’s syndrome. Fewer than 5% of those dogs have diabetes mellitus. In contrast, the serum cholesterol concentration was increased in only 13 of the 43 cats with FCS (30%), and all 13 had concurrent diabetes mellitus. Diabetes mellitus and hyperadrenocorticism are two of the classic causes for increases in serum cholesterol concentration. Excess serum cortisol concentrations also cause negative feedback to the pituitary, thereby decreasing TSH secretion and resulting in secondary hypothyroidism. Hypothyroidism is another classic cause of hypercholesterolemia. Hypothyroidism, together with the direct lipolytic actions of glucocorticoids exaggerated in Cushing’s syndrome, are common explanations for the increases in serum cholesterol concentration typically identified in nondiabetic dogs with hyperadrenocorticism. However, serum T4 concentrations were lower than the reference range in only 4 of the 43 cats (9%) with FCS, making this explanation for hypercholesterolemia unlikely. Cats with FCS were consistently euthyroid, and evidence of thyroid disease was identified in only three cats (all were hyperthyroid) at necropsy. Although thyroid disease remains possible in any cat, it would not be considered typical of cats with FCS.
BUN, SERUM CREATININE, URINE SPECIFIC GRAVITY.
A review of the serum biochemical data presented in Table 7-8 from cats with naturally occurring FCS demonstrates remarkable differences in test results as compared with those from dogs that have Cushing’s syndrome. None of the 43 cats had a BUN concentration below the reference range, and only 1 of the 43 had a serum creatinine concentration below the reference range. Only 1 of 43 cats had a randomly obtained urine specific gravity <1.020. That cat had both isosthenuria and renal failure. Furthermore, 23 of the 43 cats (53%) had abnormally increased BUN concentrations at the time of diagnosis, and 14 of those 23 cats (14 of the total 43 = 32%) also had abnormally increased serum creatinine concentrations. Thus it is likely that the polyuria and polydipsia recognized in most cats with FCS is either due to diabetes mellitus, chronic renal failure, or a combination of both conditions.
BLOOD GLUCOSE CONCENTRATIONS.
Perhaps the most unpredictable routine biochemical test result, as compared with expectations, was the random blood glucose concentration. Since a majority of cats diagnosed with FCS have diabetes mellitus, increases in blood glucose concentration are expected. However, of the 43 cats with serum biochemical test results available at the approximate time of diagnosis, 10 cats were not diabetic. Of the 33 cats with diabetes mellitus whose serum chemistry results were available for inclusion in Table 7-8, 32 were being treated with exogenous insulin at the time of FCS diagnosis. It is not surprising, then, that six of the insulin-treated cats demonstrated hypoglycemia on random blood glucose testing. It is somewhat surprising, however, that only 2 of all 43 cats with FCS had blood glucose concentrations within reference limits. Furthermore, one of those two cats was diabetic and had received insulin prior to being tested that day. Ten of 11 FCS cats that had not been treated with insulin, therefore, demonstrated hyperglycemia when blood chemistry results were randomly obtained during the hospitalization in which the FCS was diagnosed. Therefore it does seem reasonable to suggest that finding a blood glucose concentration within reference limits is unusual in cats diagnosed with Cushing’s syndrome.
HYPERGLYCEMIA.
Of the 43 cats with FCS whose laboratory data were included in Table 7-8, 35 were hyperglycemic at the time that blood was obtained for the chemistry profile. Twenty-six of the 35 hyperglycemic cats were diabetic, and 25 of the 26 were being treated with insulin and had received insulin that day. Since cats with FCS are commonly believed to be insulin-resistant, the finding of hyperglycemia would be expected in some of these cats. Resistance is not an acceptable explanation for all these hyperglycemic cats, however. An additional reason for the hyperglycemia could be inadequate control of the diabetes (with numerous causes ranging from owner error to FCS). Another possible explanation is that blood was obtained before or after the period of insulin effectiveness. Finally, any of these cats may have had stress-induced hyperglycemia in addition to their diabetes mellitus.
DIABETES MELLITUS.
We encourage veterinarians having difficulty controlling diabetes in any cat to prioritize the potential explanations for difficult control. The top of this priority list should be the fact that in-hospital testing may not reflect what is happening in the home environment. Owner history is the single most important monitoring tool in the long-term management of diabetes in cats, and if the owner is satisfied with the cat’s response to therapy, in-hospital test results should not supersede this opinion unless hypoglycemia is documented. In-hospital stress-induced hyperglycemia is common, and this differential diagnosis should not be limited to “mean” (or obviously stressed) cats.
ENDOCRINE SCREENING TESTS
Urine Cortisol-to-Creatinine Ratio (UC:CR)
BACKGROUND.
The urine cortisol-to-creatinine ratio (UC:CR) has been recommended as a screening test to aid in separating patients with Cushing’s syndrome from those without the condition. A thorough explanation of the test and the indications for its use are provided in Chapter 6. In summary, the “gold standard” screening test for Cushing’s syndrome in human beings has been to assess total cortisol excreted in urine over a 24-hour period. Repeating this test several times further ensures validity of results. Cortisol excreted in urine reflects that secreted by the adrenal glands over time and negates concern of minute-to-minute pulsatile fluctuations in plasma concentrations. It is considered a reliable test for understanding whether the adrenal glands are producing normal or excessive quantities of cortisol.
PUBLISHED DATA.
In the initial study evaluating the UC:CR in cats, it was suggested that there was a paradox in comparing reference ranges for dogs versus those for cats because of relative rates of urinary cortisol excretion when comparing the two species. The paradox is related to the relatively high reference UC:CR range in cats versus their low urinary excretion of administered radiolabeled cortisol. Healthy dogs excrete larger amounts of cortisol into urine than do healthy cats. It was suggested that the higher UC:CR in healthy cats, despite their lower overall excretion, may be explained by a higher glomerular filtration rate and/or a lower renal reabsorption of free cortisol. Thus the ratio between unaltered cortisol to conjugates and metabolites of cortisol in urine would be higher in cats than dogs (Goossens et al, 1995).
It is also wise to review the concerns that many veterinary clinical researchers have regarding specificity of UC:CR testing when it is used as the sole diagnostic aid for confirming Cushing’s syndrome in cats. The results from one study indicate that health status of cats affects UC:CR (Henry et al, 1996). Test results in healthy cats were not affected by age, gender, or neuter status. Ill cats have higher UC:CR values than healthy cats, implying that specificity may be a problem (as in dogs). The UC:CR from 16 ill cats were evaluated. Three cats had test results consistent with hyperadrenocorticism (≥3.6 × 10−5), and another seven had results in the range we would consider “borderline” (i.e., between 1.0 and 3.6 × 10−5. Thus 10 of 16 ill cats had UC:CR results that could be considered consistent with a diagnosis of hyperadrenocorticism. However, although the study demonstrated that sick cats had significantly higher UC:CR results than healthy cats, it did not include any results from cats with naturally occurring Cushing’s syndrome, negating direct comparison of data from each of the three groups in question (healthy, ill, and FCS cats; Henry et al, 1996).
TEST INTERPRETATION.
Two studies have been published suggesting reference ranges for feline UC:CR. The reference range from the first report was <3.6 × 10−5 (Goossens et al, 1995), and from the second report it was <2.8 × 10−5 (Henry et al, 1996). If the initial reference range for cats (<3.6 × 10−5) is used, it should be remembered that this value is slightly higher than that for dogs (<1.0 or <1.3 × 10−5). It seems prudent to suggest, from the information published in these two reports coupled with our experience, that a UC:CR value ≥3.6 × 10−5 in a cat with clinical signs consistent with hyperadrenocorticism (FCS) is consistent with that diagnosis. We also recommend that UC:CR values between 1.3 and 3.6 × 10−5 in cats with appropriate clinical signs be considered “borderline.” It is possible for a cat with test results in this range to have naturally occurring hyperadrenocorticism.
RESULTS.
As can be seen from the data presented in Tables 7-9 and 7-10, we were able to collate UC:CR data from a total of 37 cats that had confirmation of their naturally occurring hyperadrenocorticism. Twenty-four of the cats were from our series, and 13 were from cats from the literature with well-documented FCS (Goossens et al, 1995 [6 cats]; Meij et al, 2001 [7 cats]). Of these 37 cats, 29 had PDH and 8 had functioning adrenocortical tumor hyperadrenocorticism (ATH). Twenty-nine of the total of 37 cats (78%; 24 from the PDH group and 5 from the ATH group) had results above the reference range of 3.6 × 10−5. An additional 7 cats (19%) had results in the borderline range (4 from the PDH group and 3 from the ATH group). This now accounts for results from all 8 cats with ATH and for 28 of the 29 cats with PDH. Borderline values were defined as those above the approximate canine reference range (<1.3 × 10−5) but lower than the higher range established for cats in the previously mentioned study (<3.6 × 10−5). Only one cat had a UC:CR test result that was considered normal (that cat had PDH). Thus the UC:CR appears to be a sensitive test for confirming the diagnosis of Cushing’s syndrome in cats, with a sensitivity approaching that demonstrated for dogs. We have not evaluated the specificity of the UC:CR but suspect that it may have similar problems to those noted in dogs.
TABLE 7-9 ENDOCRINE SCREENING TEST RESULTS FROM CATS WITH NATURALLY OCCURRING PITUITARY-DEPENDENT CUSHING’S SYNDROME
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree