Kelly L. Carlson
Hepatic Diseases in the Horse
The liver is one of the largest organs in the equine body and is responsible for multiple functions. It regulates nutrient metabolism, distribution, and homeostasis and is responsible for the synthesis, storage, and release of glucose. The liver also excretes bile, metabolizes and detoxifies substances, and synthesizes proteins, such as albumin, fibrinogen, and clotting factors. Hepatic dysfunction is relatively common in adult horses and occasionally occurs in foals.
Clinical Signs
Signs of hepatic dysfunction are often nonspecific and depend on the severity and duration of hepatic disease. Typically, 80% or more of the liver must be damaged before clinical signs become apparent. Common clinical signs of hepatic dysfunction in the horse include depression, anorexia, colic, and weight loss. Signs more specific for liver disease include icterus, photosensitization, and hepatic encephalopathy. Hepatic encephalopathy (HE) may manifest in multiple ways, including depression, aggression, yawning, circling or walking compulsively, or head pressing (Figure 66-1). Bilateral laryngeal paralysis, gastric impactions, and abdominal tenesmus have been reported as rare complications in horses with hepatic encephalopathy.
Diagnosis
Biochemical Tests
Standard biochemical indices of hepatocellular disease include sorbitol dehydrogenase (SDH), aspartate aminotransferase (AST), and lactate dehydrogenase (LDH). SDH is the most specific indicator of acute hepatocellular damage in the horse. However, SDH quantification cannot be performed at all diagnostic laboratories. The enzyme is stable at room temperature for less than 12 hours, so serum or plasma should be separated and frozen if quantification cannot be performed in a timely fashion. SDH has a short half-life (several hours), so high values indicate active hepatocellular damage. Foals younger than 1 month may have higher SDH values than adults. The other hepatocellular enzymes, AST and LDH, are frequently measured in horses. AST is poorly specific for hepatic damage and is commonly released from damaged myocytes and erythrocytes. AST has a much longer half-life (7 to 8 days) than SDH, and high values may persist for a week or more past the resolution of hepatocellular damage. Similarly, LDH is poorly specific because it also is released from muscle. Unlike AST, LDH has a short half-life (<24 hours). High values for SDH, AST, and LDH do not indicate a specific liver disease, although elevations are more likely with hepatocellular conditions.
Standard biochemical indicators of hepatobiliary disease include γ-glutamyl transferase (GGT) and alkaline phosphatase (ALP). GGT is a specific indicator of biliary epithelial damage and is the most sensitive indicator of hepatic disease in horses. GGT is released into the circulation as a result of biliary damage, biliary hyperplasia, or cholestasis. Serum GGT activity has been reported to be high in 49% of horses with right dorsal displacement of the large colon, presumably secondary to transient obstruction of the bile duct. GGT has a moderately long half-life (3 to 4 days), and values may remain high for days to weeks beyond resolution of the inciting cause. Foals younger than 1 month may have higher GGT values than adults. ALP is released from multiple sites, including liver, bone, and intestine, so elevations are not specific for liver disease. ALP may be increased with cholestasis or chronic liver disease. Foals normally have ALP values two to three times those of adults because of osteoblastic activity associated with growth. High GGT and ALP values do not indicate a specific liver disease, but high values are more likely with hepatobiliary disease.
Serum bile acid concentrations may be consistently high with either hepatocellular or hepatobiliary disease. Fasting is not required for accurate serum bile acid determination in horses. Bile acid concentrations may increase secondary to cholestasis (decreased bile acid excretion from the liver) or decreased functional hepatic mass (decreased bile acid clearance from portal blood). Bile acids tend to be higher with hepatobiliary disease than with hepatocellular disease.
Serum or plasma bilirubin concentration can be high in horses with any type of liver disease. Bilirubin is evaluated as unconjugated (indirect), conjugated (direct), or total bilirubin. Mild hyperbilirubinemia (increased total and unconjugated bilirubin) develops in horses that are anorexic or feed-restricted. A marked increase in unconjugated bilirubin can occur secondary to severe hepatic disease or hemolysis. Elevations in the conjugated fraction (especially if greater than 25% of the total bilirubin) indicate cholestatic or hepatobiliary disease.
Blood ammonia levels may also be evaluated as an indicator of hepatic function. Samples for blood ammonia must be kept chilled and rapidly evaluated; therefore this testing option is not available in all clinical settings. Ideally, a control sample from a clinically healthy horse should be evaluated in tandem with the clinical case. High blood ammonia levels may be seen secondary to decreased functional hepatic mass (decreased clearance of NH4+ from portal blood) or portosystemic shunt (portal blood never reaches the liver for NH4+ removal). A congenital defect in the urea cycle has led to hyperammonemia in Morgan foals. Hyperammonemia without concurrent liver disease can also occur with acute gastrointestinal disease (see Chapter 139).
Hepatic Ultrasound
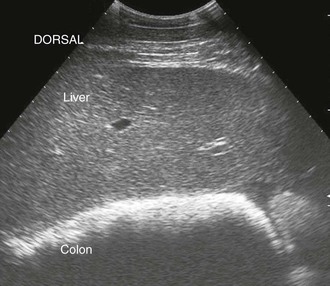
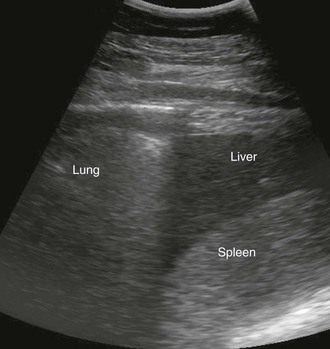
Transabdominal ultrasound of the liver is a valuable diagnostic tool for further evaluation of horses with clinical signs and clinicopathologic abnormalities consistent with hepatic dysfunction. Hepatic ultrasonography is safe, is noninvasive, and provides information regarding the general size, shape, and location of the liver. Additionally, ultrasonography can detect changes in the hepatic parenchyma and biliary structures. Ultrasound is also helpful for selecting an appropriate location for hepatic biopsy. The normal equine liver is ultrasonographically recognizable by its branching vasculature, echogenic portal vein walls, and homogenous, medium-echogenicity parenchyma (Figure 66-2). The hepatic parenchyma is generally more echogenic than the renal cortex and medulla but less echogenic than the spleen (Figure 66-3) and renal pelvis.
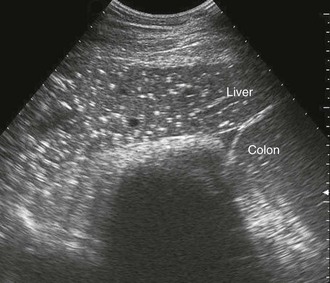
Multiple abnormalities of the equine liver have been described on the basis of ultrasonographic appearance. Disorders of the liver may cause a change in the size of the liver, such as a small liver with hepatic fibrosis or an enlarged liver (indicated by rounded ventral margin) with neoplasia. Focal hepatic lesions have been reported in horses with hydatid cysts, polycystic liver disease, hepatic abscesses, cholelithiasis, and neoplasia. Diffuse hepatic lesions have been described in horses with the starry sky liver pattern (Figure 66-4). Changes in parenchymal echogenicity have been described with hepatic necrosis or fibrosis, hepatic lipidosis, granulomatous liver disease, hepatic amyloidosis, and cholangiohepatitis. Changes in the biliary tract, including cholelithiasis and biliary distension, have also been described.
The adult equine liver can be imaged with a 2.5- or 5-MHz transducer. Neonatal foals may be imaged with a 7.5- or 10-MHz transducer. In both adults and foals, the largest portion of the liver is imaged on the right side of the abdomen, immediately ventral and caudal to the right lung, from the 6th to 15th intercostal space. A smaller portion of the liver can be imaged in the cranioventral aspect of the left side of the abdomen, adjacent to the spleen, from the 6th to 9th intercostal space. In older horses, the right liver lobe atrophies, reducing the amount of liver visible in the right side of the abdomen. In addition, the extent of lung fields, gas distension of the colon, and splenomegaly can obscure the liver.
Hepatic Biopsy
Liver biopsy is often required for a definitive diagnosis in horses with evidence of hepatic disease. The history, clinical signs, and clinicopathologic abnormalities may indicate a presumptive diagnosis, but biopsy is required to determine the extent and severity of disease, which is important for determining prognosis. The standard anatomic landmark for liver biopsy is the intersection between a line drawn from the point of the shoulder to the tuber coxae and the 14th intercostal space. However, liver tissue may not be obtained from this space in all horses, and there is significant risk for obtaining a biopsy of the colon, lung, or diaphragm. Therefore use of ultrasound for selection of an appropriate biopsy location and possibly for guidance of the biopsy instrument is recommended.
Evaluation of a coagulation profile is recommended before hepatic biopsy. A control sample from a normal horse should be evaluated at the same time for quality control purposes. Although subclinical coagulopathy has been reported in horses with liver disease, clinically significant or fatal hemorrhage after a liver biopsy is rare. Ultrasonographic evaluation can be useful to monitor for significant hemorrhage following a biopsy. This author recommends a 14-gauge Tru-Cut biopsy needle (multiple distributors) or a 14-gauge automated biopsy instrument1 to obtain sufficient material for histologic evaluation. Smaller-gauge devices generally yield insufficient material.
Sufficient liver material should be obtained for both cytologic examination and culture. Cytologic examination is generally most important if the available liver tissue is limited. Important cytologic findings include predominant cellular populations of inflammatory infiltrate and the presence and extent of periportal or bridging fibrosis. Biliary hyperplasia may occur with nearly every hepatocellular or hepatobiliary disease. The presence and type of inflammatory infiltrate may help direct treatment, and the presence and severity of fibrosis indicates prognosis. Generally speaking, the presence of mature collagen that fully bridges portal tracts is a poor prognostic sign. Minimal or absent fibrosis confers a more favorable prognosis.
Differential Diagnosis
Distinguishing between acute and chronic liver disease is often a challenge, particularly given the wide variety of clinical signs. Furthermore, most of the hepatic parenchyma must be lost before function is affected, so even a sudden onset of signs may actually result from chronic disease. The hallmark evidence of chronicity is the presence of fibrosis, which can only be identified histologically. For the purposes of discussion, diseases have been divided into acute or chronic based on the known cause of the disease.
Acute Liver Disease
Theiler’s Disease
Theiler’s disease (acute hepatitis necrosis, serum-associated hepatitis, serum sickness) has been reported in horses for nearly 100 years, but the exact cause remains unknown. Theiler’s disease is considered to be one of the most common causes of acute hepatic failure. The disease typically occurs in individual horses, but outbreaks of multiple horses have occurred. Generally, horses with Theiler’s disease have a history of receiving an equine-origin biologic antiserum in the 4 to 10 weeks before the onset of signs. Biologic products that have been implicated include vaccines, antisera, tetanus antitoxin, and plasma. Some reports suggest that lactating mares that receive tetanus antitoxin after parturition are particularly prone to Theiler’s disease. Other horses with Theiler’s disease have no history of receiving biologics. Horses present with an acute onset of signs of hepatic failure, including anorexia, icterus, and signs of HE. Histopathologic changes in affected liver consistently include widespread centrilobular to midzonal hepatocellular necrosis with hemorrhage. There is no specific treatment aside from general supportive care. The prognosis is poor to grave, although horses that survive longer than 1 week often recover.
Bacterial Hepatitis
Tyzzer’s disease, caused by Clostridium piliforme, is the most common cause of bacterial hepatitis in foals. It is confined to foals between 7 and 42 days of age, which are often found acutely dead or with nonspecific signs of enteritis and hepatitis. C piliforme is found in the feces of healthy adult horses as well as in the environment. Foals are infected by ingesting contaminated soil or feces. The organism causes acute multifocal hepatitis and enteritis and can be identified on histologic examination of the liver. Tyzzer’s disease is highly fatal, although rare reports exist of successful treatment with antimicrobials and supportive care.
Infectious necrotic hepatitis (black disease), caused by infection with Clostridium novyi type B, has rarely been reported in horses. Infection has occurred in horses that reside in or adjacent to areas with a large population of sheep. The infection usually proves fatal, and the carcass blackens rapidly after death secondary to engorgement of subcutaneous blood vessels (giving rise to the name black disease). Additional causes of bacterial hepatitis are rare in adult horses. Neonates may develop bacterial hepatitis secondary to septicemia. Supportive care and antimicrobial therapy are the mainstays of treatment.
Bacterial cholangiohepatitis is diagnosed in horses, typically secondary to cholestasis, cholelithiasis, or some other ascending infection from the gastrointestinal tract. Definitive diagnosis is made on the basis of histopathologic changes and bacterial isolation from a liver biopsy specimen. Isolates are usually enteric organisms, such as Salmonella spp, Escherichia coli, Klebsiella spp, and Acinetobacter spp. Treatment includes supportive care and long-term antimicrobial therapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree