Simon F. Peek
Hemolytic Disorders
Hemolysis is defined as premature destruction of red blood cells (RBCs). This destruction can occur either within the vasculature (intravascular hemolysis) or in other tissues or organs (extravascular hemolysis), most commonly within the reticuloendothelial infrastructure of the spleen or liver. Intravascular hemolysis causes release of hemoglobin directly into the plasma, where it becomes bound by haptoglobin. The quantity of free plasma haptoglobin therefore decreases during conditions causing intravascular hemolysis in direct proportion to the amount of hemoglobin being released through erythrocyte lysis. Haptoglobin is synthesized in the liver, and its production is upregulated during proinflammatory states, such that the level of available plasma haptoglobin to interact with free hemoglobin depends on biosynthetic liver function and concurrent inflammatory stimuli. With excessive release of hemoglobin into the circulation, saturation of haptoglobin binding leads to free hemoglobin in the plasma (hemoglobinemia). This hemoglobin is filtered by the kidneys and reabsorbed in the proximal tubules, where it is catabolized in situ. When the absorptive capacity of the proximal tubules is exceeded, hemoglobinuria results. Hemoglobinuria is typically only seen in horses subsequent to severe acute intravascular hemolysis, which can be differentiated from the more common pigmenturia associated with myoglobin release by the persistence of a brownish-red tinge to the plasma following centrifugation. Hemoglobin has a higher molecular weight than myoglobin, making it less readily filtered by the glomeruli and persistent in the circulation for much longer. It is worth remembering that on urinalysis, hemoglobinuria, myoglobinuria, and hematuria all give a positive reaction with the commonly used benzidine reaction. Haptoglobin–hemoglobin complexes are themselves removed by the reticuloendothelial system, especially in the liver, for further catabolism. The normal circulatory life span of the equine erythrocyte is reported to be 155 days, which is considerably longer than that in other veterinary species and humans.
Horses with clinically relevant hemolysis typically have substantial anemia that can be confirmed by peripheral blood analysis. Mild hemolytic disease may be clinically occult and not associated with anemia on analysis. Anticoagulated blood samples from horses with hemolytic anemia do not reliably contain evidence of hematologic regeneration. However, it is rarely appropriate to obtain bone marrow biopsy specimens during evaluation of hemolytic horses for proof of RBC regeneration. Physical examination findings that accompany intravascular hemolytic disease may include icterus and discolored urine secondary to hemoglobinuria but will otherwise vary according to the severity of the anemia and the degree of resultant cardiopulmonary compensation. Tachycardia and tachypnea are reflexive with the degree of hypoxemia resulting from anemia. Pyrexia can be anticipated with infectious causes but may also develop with noninfectious hemolytic etiologies because of nonspecific pyrogen release from erythrocyte lysis. Pigment nephropathy is a common and potentially life-threatening complication of acute hemolytic crises that should be anticipated and addressed in horses with piroplasmosis and acute toxic hemolytic disease. It is far less likely that horses with immune-mediated hemolytic disease or more chronic extravascular hemolysis will experience renal disease sequelae. It is prudent to establish the presence and severity of azotemia during hemolytic crises and address crystalloid therapy accordingly, being mindful that some horses may already be oliguric, or even anuric, at the time of initial evaluation. Acute intravascular hemolysis is commonly accompanied by increases in mean corpuscular hemoglobin concentration. Peripheral blood smears from horses with hemolytic disease should be carefully evaluated by trained pathologists for the presence of parasites and inclusions indicative of infectious agents associated with hemolysis, such as Babesia caballi, Theileria equi, and Anaplasma phagocytophilum. Serologic tests (immunofluorescent assay for A phagocytophilum and complement fixation tests for Babesia spp) can also be used but are indicative of infection rather than being specific for clinical disease. Polymerase chain reaction tests for the causative agents of piroplasmosis and anaplasmosis are also now widely available to confirm infection. Coombs’ test is of doubtful specificity in suspected cases of immune-mediated hemolytic anemia because several infectious causes of hemolysis in adult horses induce a transient positive test result with Coombs’ reagents.
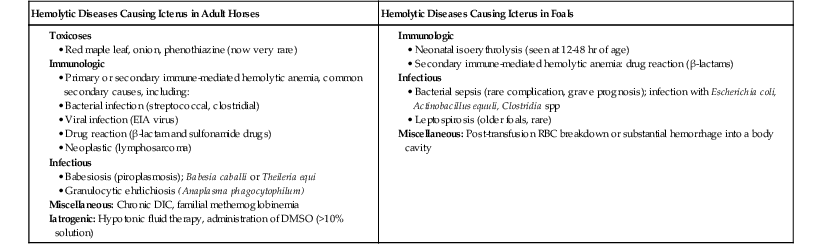
Hemolytic diseases in horses fall into three broad categories: infectious, toxic, and immunologic, and each of these will be considered in this chapter (Box 117-1).
Infectious Causes of Hemolysis
The most important infectious cause of hemolysis in horses worldwide is piroplasmosis (babesiosis), caused by one or other of the intraerythrocytic protozoans, Theileria equi (previously known as Babesia equi) and Babesia caballi. The United States, Canada, Australia, New Zealand, and much of Europe are not considered endemic areas for piroplasmosis. However, there are tick species capable of acting as vectors for the organisms in some of these areas. Outbreaks of piroplasmosis in the United States have historically been limited to Florida, typically in either imported horses or horses in contact with infected imported horses, and the major mode of transmission has been contaminated needles and other poor husbandry practices rather than traditional insect vectors.
Multiple infectious diseases can give rise to hemolysis as an occasional clinical complication, although the pathogen concerned is not considered a primary cause of hemolysis in the manner of a true erythrocytic parasite. Examples of such conditions include equine infectious anemia (EIA), equine viral arteritis (EVA), equine trypanosomiasis, and anaplasmosis (previously known as equine granulocytic ehrlichiosis). The mechanism of hemolysis in these conditions is not always well understood, but involves enhanced hemophagocytosis in the bone marrow and spleen. It is unclear whether these events in diseases such as EIA, anaplasmosis, and EVA actually represent a form of immune-mediated erythrocyte destruction or removal. Some horses with severe inflammatory conditions and resulting severe manifestations of the systemic inflammatory response syndrome may also develop hemolysis, potentially as a late-stage or near-death sequela. Severe colitis and pleuropneumonia are two such conditions that can give rise to this event. Terminally, the occasional horse with fulminant hepatic failure or, less commonly, renal failure may also experience an acute hemolytic crisis. Hemolytic uremic syndrome is a severe, life-threatening condition that is well documented in humans, especially children, in association with specific strains of Escherichia coli, but this appears to be rare in horses. However, one report described isolation of a strain of E coli 0103:H2 carrying the gene for the Shiga toxin-1 in a postpartum mare with metritis, and a small retrospective report of two cases and an additional case report involving a single horse with clinical and histopathologic signs suggestive of the condition have been published, although there was no microbiologic confirmation of the etiology in the latter two studies.