Chapter 2 Hematology Procedures
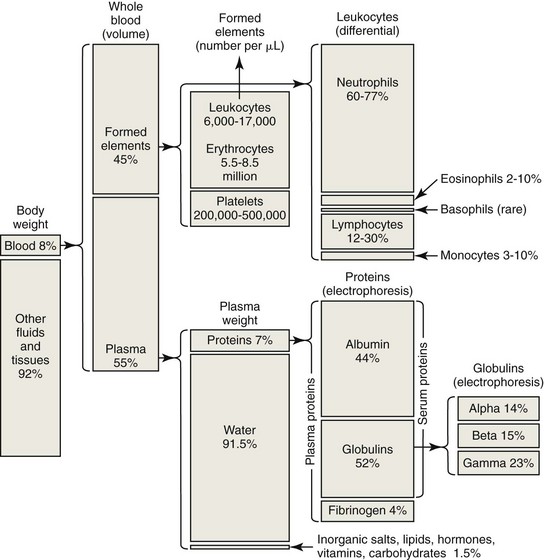
Composition of Blood
Blood is composed of cells (erythrocytes, leukocytes, and platelets) circulating within fluid called plasma (Fig. 2-1). Erythrocytes or red blood cells are most numerous, with several million erythrocytes per microliter of blood in mammals (Appendix Table 1). Depending on the species, erythrocytes typically account for one-fourth to one-half of the total blood volume as measured by determining the hematocrit. Platelets or thrombocytes are the next most numerous cell type in blood, with platelet counts as low as 100 × 103/µL in healthy horses to several hundred thousand per microliter in other mammalian species. Total leukocyte or white blood cell counts are much lower than erythrocyte and platelet counts, with total leukocyte counts ranging from about 5 × 103/µL to about 20 × 103/µL in mammals. The proportion of leukocyte types present varies by species, with neutrophils being the most numerous leukocyte type present in the blood of carnivores and lymphocytes being the most numerous leukocyte type present in the blood of ruminants and rodents.
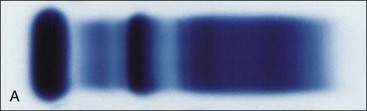
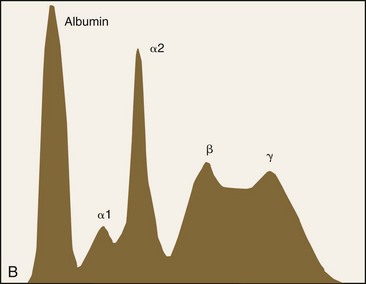
Plasma consists primarily of water that contains about 6 to 8 g/dL of plasma proteins and 1.5 to 2.0 g/dL of inorganic salts, lipids, carbohydrates, hormones, and vitamins.19 Plasma is prepared in the laboratory by collecting blood with an anticoagulant, followed by centrifugation to remove the blood cells. If blood is collected without anticoagulant and allowed to clot, the fluid that is obtained following centrifugation is called serum. The protein concentration in serum is usually about 0.2 to 0.5 g/dL lower than that in plasma, primarily owing to the absence of fibrinogen—which is consumed during coagulation—in serum. Serum proteins may be separated by electrophoresis into albumin, α-globulins, β-globulins, and γ-globulins (Fig. 2-2). Albumin is a single protein that generally accounts for nearly half of the total plasma proteins present by weight. Each of the globulin classes is composed of many different proteins.12
Calculation of Blood Volume
Total blood volume accounts for about 10% to 11% of body weight in hot-blooded horses; 8% to 9% in dogs; 6% to 7% in cats, ruminants, laboratory rodents, and cold-blooded (draft) horses; and 5% to 6% in pigs. The total blood volume of young growing animals often exceeds 10% of body weight.33 It may be desirable to calculate the total blood volume of an animal in determining the size of a needed blood transfusion, or the amount of blood that can safely be removed for a series of diagnostic tests, or when an animal is to be used as a blood donor. For example, the total blood volume of a 4-kg cat is 0.07 × 4 kg = 0.28 kg = 280 mL, assuming that 7% of body weight is blood in cats and the specific gravity of blood is 1.0 (1 mL weighs 1 g). Since one can safely remove 20% of the blood volume from an animal, the calculated amount that can be removed from the cat in this example is 280 × 0.2 = 56 mL.
Sample Collection and Handling
In monogastric animals, an overnight fast avoids postprandial lipemia, which can interfere with plasma protein, fibrinogen, and hemoglobin determinations. Ethylenediaminetetraacetic acid (EDTA) is the preferred anticoagulant for complete blood count (CBC) determination in most species, but blood from some birds and reptiles hemolyzes when collected into EDTA. In those species, heparin is often used as the anticoagulant. The disadvantage of heparin is that leukocytes do not stain as well (presumably because heparin binds to leukocytes),24 and platelets generally clump more than they do in blood collected with EDTA. However, as discussed later, platelet aggregates and leukocyte aggregates may occur even in properly collected EDTA-anticoagulated blood samples.10,29,41,49,53 In those cases, collection of blood using another anticoagulant (e.g., citrate) may prevent the formation of cell aggregates. Cell aggregation tends to be more pronounced as blood is cooled and stored; consequently processing samples as rapidly as possible after collection may minimize the formation of leukocyte and/or platelet aggregates.
Gross Examination of Blood Samples
Methemoglobinemia
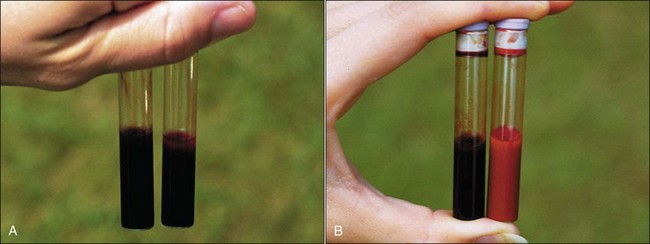
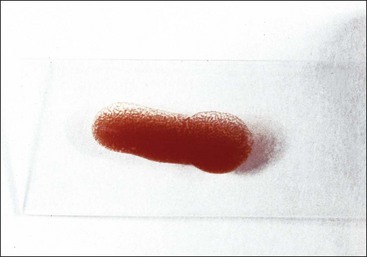
Hemoglobin is a protein consisting of four polypeptide globin chains, each of which contains a heme prosthetic group within a hydrophobic pocket. Heme is composed of a tetrapyrrole with a central iron molecule that must be maintained in the ferrous (+2) state to reversibly bind oxygen. Methemoglobin differs from hemoglobin only in that the iron molecule of the heme group has been oxidized to the ferric (+3) state and is no longer able to bind oxygen.28 The presence of large amounts of deoxyhemoglobin accounts for the dark bluish color of normal venous blood samples. Methemoglobinemia may not be recognized in venous blood samples because the brownish color of methemoglobin is not readily apparent when methemoglobin is mixed with deoxyhemoglobin (Fig. 2-3, A). When deoxyhemoglobin binds oxygen to form oxyhemoglobin, it becomes bright red; consequently the brownish coloration of methemoglobin becomes more apparent in the oxygenated samples (Fig. 2-3, B). A simple spot test provides a rapid way to oxygenate a venous blood sample and to determine whether clinically significant levels of methemoglobin are present. One drop of blood from the patient is placed on a piece of absorbent white paper and a drop of normal control blood is placed next to it. If the methemoglobin content is 10% or greater, the patient’s blood will have a noticeably brown color compared with the bright red color of control blood (Fig. 2-4).28 Accurate determination of methemoglobin content requires that blood be submitted to a laboratory that has this test available. Methemoglobinemia results from either increased production of methemoglobin by oxidants or decreased reduction of methemoglobin resulting from a hereditary deficiency in the erythrocyte cytochrome-b5 reductase enzyme (see Chapter 4).27
Erythrocyte Agglutination
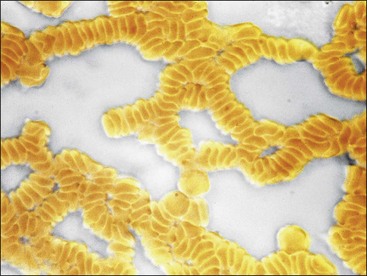
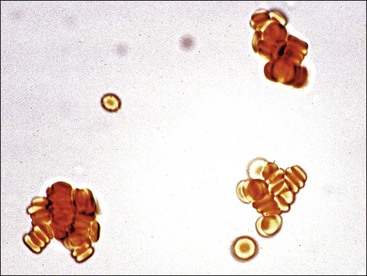
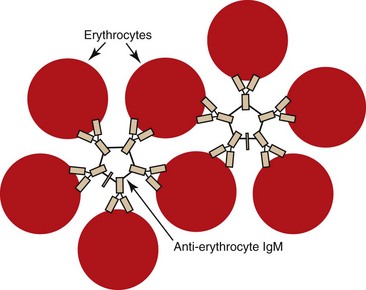
The appearance of red granules in a well-mixed blood sample (Fig. 2-5) suggests the presence of erythrocyte autoagglutination. However, it is important to differentiate agglutination (aggregation of erythrocytes together in clusters) from rouleaux (adherence of erythrocytes together like a stack of coins), which can be seen in the blood from healthy horses and cats (Fig. 2-6). Rouleaux formation is eliminated by washing erythrocytes in physiologic saline, but agglutination is not. This differentiation requires centrifugation of blood, removal of plasma, and resuspension of erythrocytes in saline. A rapid way to differentiate rouleaux from agglutination is to mix five drops of physiologic saline with a drop of anticoagulated blood on a glass slide and examine it as a wet mount using a microscope. This dilution reduces rouleaux, but agglutination is not affected. The microscopic appearance of agglutination in a sample of washed erythrocytes is shown (Fig. 2-7). The presence of agglutination indicates that the erythrocytes have increased surface-bound immunoglobulins. These immunoglobulins are usually of the IgM type when agglutination is present, because the greater distance between binding sites on IgM molecules compared with IgG molecules makes it easier for IgM molecules to overcome normal repulsive forces between negatively charged erythrocytes.67 In addition, there are 10 antigen-binding sites per IgM molecule compared with 2 binding sites per IgG molecule (Fig. 2-8). A direct antiglobulin test is not needed to identify the presence of immunoglobulin bound to erythrocytes if agglutination is present in saline solution-washed erythrocyte samples.
Microhematocrit Tube Evaluation
Packed Cells
The PCV is measured after centrifugation by determining the fraction of total blood volume in a microhematocrit tube that is occupied by erythrocytes. Leukocytes and platelets are primarily located within the buffy coat, although certain leukocyte types may be present in the top portion of the packed erythrocyte column in some species (e.g., neutrophils in cattle). The width of the buffy coat generally correlates directly with the total leukocyte count. A large buffy coat suggests leukocytosis (Fig. 2-9, A) or thrombocytosis, and a small buffy coat suggests that low numbers of these cells may be present. The buffy coat may appear reddish owing to the presence of a marked reticulocytosis.
The Appearance of Plasma
Plasma is normally clear in all species. It is nearly colorless in small animals, pigs, and sheep but light yellow in horses because they naturally have higher bilirubin concentrations. Plasma varies from colorless to light yellow (carotenoid pigments) in cattle, depending on their diet.62 Increased yellow coloration usually indicates increased bilirubin concentration. This increase often occurs secondarily to anorexia (fasting hyperbilirubinemia) in horses owing to reduced removal of unconjugated bilirubin by the liver.13 Failure of the liver to remove unconjugated bilirubin from blood has also been reported to cause hyperbilirubinemia in one-fourth of the sick (often anorectic) cattle in one study.40 In other species, yellow plasma with a normal HCT suggests hyperbilirubinemia secondary to liver disease. Hyperbilirubinemia associated with a marked decrease in the HCT suggests an increased destruction of erythrocytes; however, the concomitant occurrence of liver disease and a nonhemolytic anemia could produce a similar finding (Fig. 2-9, B).
Red discoloration of plasma indicates the presence of free hemoglobin. This discoloration may represent either true hemoglobinemia, resulting from intravascular hemolysis, or hemolysis occurring after sample collection due to such causes as rough handling, fragile cells, lipemia, or prolonged storage (Fig. 2-9, C,D). The HCT value may help differentiate between these two possibilities, with red plasma and a normal HCT suggesting in vitro hemolysis. The concomitant occurrence of hemoglobinuria indicates that intravascular hemolysis has occurred. Plasma will also appear red following treatment with cross-linked hemoglobin blood substitute solutions.
Lipemia is recognized as a white opaque appearance caused by chylomicrons and very low density lipoproteins (VLDLs). The presence of chylomicrons may also result in a white layer at the top of the plasma column (Fig. 2-9, E). The presence of lipemia is frequently the result of a recent meal (postprandial lipemia), but diseases including diabetes mellitus, pancreatitis, hypothyroidism, hyperadrenocorticism, protein-losing nephropathy, cholestasis, obesity, and starvation may also contribute to the development of lipemia in dogs and cats.23,72 Transient lipemia and accompanying anemia has been described as a syndrome in kittens, but a direct link between hyperlipidemia and anemia has not been clearly documented.23 Hereditary causes of lipemia include lipoprotein lipase deficiency in cats and idiopathic hyperlipidemia in miniature schnauzer dogs.20,73
Equids of any age and either sex may develop lipemia, but obese ponies, miniature horses, and miniature donkeys are most susceptible. Lipemia has been associated with a broad range of diseases, but it is more prevalent in association with pregnancy, lactation, and/or anorexia.65 Lipemia has also been reported in llamas and alpacas with severe systemic diseases. It is not associated with age, sex, or reproductive status in these camelids.64 The pathogenesis of marked secondary hyperlipidemia is not always clear. It often appears to result from a mobilization of unesterified fatty acids from adipose tissue and the subsequent overproduction of VLDLs by the liver, but it may also result from ineffective clearance of VLDLs by tissues or a combination of both, as occurs in diabetes mellitus.
Plasma Protein Determination
After the PCV is measured and the appearance of the plasma and buffy coat is noted, the microhematocrit capillary tube is broken just above the buffy coat and the plasma is placed in a refractometer for plasma protein determination. Plasma protein concentrations in newborn animals (approximately 4.5 to 5.5 g/dL) are lower than adult values and increase to within the adult range by 3 to 4 months of age.33 The presence of lipemia or hemolysis will falsely increase the measured plasma protein value. Maximum information can be gained by interpretation of the HCT and plasma protein concentrations simultaneously (see Chapter 4).
Fibrinogen Determination
Fibrinogen can be measured in a hematocrit tube because it readily precipitates from plasma when heated to 56°C to 58oC for 3 minutes. The difference between the total protein of the plasma and the total protein of the defibrinogenated (heated) plasma gives an estimate of the fibrinogen concentration in the plasma.32 This method is useful in identifying high fibrinogen concentrations, but it is not accurate in identifying low fibrinogen concentrations.4,11
Blood Cell Counting and Sizing
Manual Cell Counting
All blood cell types in birds and reptiles are nucleated, making accurate separation and counting difficult or impossible with automated cell counters. Consequently manual leukocyte counts are generally required in nonmammalian species. Thrombocytes can be estimated based on the number present in stained blood films.47
Automated Cell Counting
Quantitative buffy coat analysis (QBC VetAutoread Hematology System, IDEXX, Inc., Westbrook, ME) depends on variations in cell density to separate cell types. Cells are not actually counted; instead the widths of the various layers of cell types that form are measured and the cell “count” is generated assuming a standard cell size for the cell type in question.35
The development of laser flow cytometry for use in instruments such as the CELL-DYN 3500R (Abbott Laboratories, Abbott Park, IL), the Advia 120 (Siemens Healthcare Diagnostics, Inc., Tarrytown, NY), the Sysmex XT-2000iV (Sysmex Corporation, Kobe, Japan), and the LaserCyte (IDEXX, Inc., Westbrook, ME) has allowed for cells to be characterized in greater detail. Individual cells pass through a laser beam, absorbing and scattering light. Interruptions in light are used to count cells and light scatter is used to determine size and internal complexity or density. The Advia 120 also utilizes a peroxidase channel to aid in determining specific leukocyte types. When properly calibrated, laser flow analysis allows for a more complete and accurate differential leukocyte count than can be done using cell size alone. Laser flow cytometry also permits the development of automated reticulocyte counts and reticulated platelet counts36,44 as well as the development of new erythrocyte and reticulocyte parameters based on the simultaneous measurement of size and hemoglobin concentration within individual cells.17
Errors in Blood Cell Counting and Sizing
The accuracy of blood cell counting depends on the quality and characteristics of the blood sample as well as the accuracy of the analytic methods used. Storage of blood samples for more than a few hours can result in sample deterioration and inaccurate cell counts. The presence of even small clots in the blood tube invalidates all cell counts. Even without clot formation, platelet aggregation may occur if platelets become activated during blood collection, as can happen when specimens are collected with a syringe and then transferred to an anticoagulant tube. Heparin is generally not used as an anticoagulant because it does not prevent platelet clumping, and leukocyte staining is poor on blood films. EDTA is the preferred anticoagulant for CBC determinations in most species, but EDTA may induce platelet, leukocyte, and erythrocyte aggregate formation in some individuals with antibodies bound to the surfaces of these respective cell types.10,29,54,71,75 When this occurs, collection of blood into citrate may prevent the problem. Cell aggregation tends to be more pronounced as blood is cooled and stored; consequently processing samples as rapidly as possible after collection may minimize the formation of leukocyte and/or platelet aggregates. EDTA can cause marked hemolysis in some species of birds (ostriches, emus, and jays),25,35 reptiles (turtles and tortoises),25,42 and fish (carp and brown trout).43,66 Heparin is used as an anticoagulant in these species. Both lysis and clumping can result in reduced cell counts of the cell types involved.
Platelet clumping can not only result in an erroneously decreased platelet count59 but also in a falsely increased mean platelet volume (MPV) and occasionally a falsely increased total leukocyte count.35,75 Autoagglutination of erythrocytes in the blood sample can result in a spuriously increased mean cell volume (MCV) and mean cell hemoglobin concentration (MCHC) and a decreased HCT and total erythrocyte count.48 As indicated earlier, with the use of most hematology analyzers, the presence of nucleated erythrocytes can result in a falsely increased total leukocyte count. Residual erythrocyte stroma from inadequate lysing of erythrocytes may also result in spuriously increased total leukocyte counts.35,76
The presence of severe lipemia can result in spuriously increased hemoglobin and MCHC values and possibly even increased platelet and total leukocyte counts.75 The presence of in vitro or in vivo hemolysis in the sample or prior treatment with a cross-linked hemoglobin solution can result in an erroneously increased MCHC value.38 The presence of numerous Heinz bodies in erythrocytes can also result in spuriously increased hemoglobin and MCHC values, increased automated reticulocyte counts,14,52 and sometimes increased total leukocyte counts.69 The precipitation of an IgM paraprotein in blood from a dog by the lysing reagent used in the CELL-DYN 3500 falsely increased the spectrophotometric measurement of hemoglobin (Hb), which falsely increased the calculated MCHC.8
Erythrocytes and platelets vary considerably in volume among animal species, and instruments must be adjustable to accurately count and size these blood cells for the species being assayed. Cats naturally have large platelets and moderately small erythrocytes. The resultant overlap of erythrocyte and platelet size makes separation of cat platelets and erythrocytes unreliable with the use of impedance counters.77 Consequently cat platelet counts are spuriously decreased when measured with impedance counters. This inclusion of platelets in the erythrocyte measurements can result in increased erythrocyte counts and HCT values and reduced MCV and MCHC values, but the ratio of platelets to erythrocytes is usually not large enough to have appreciable effects on these parameters (exceptions include cats with severe anemia and/or marked thrombocytosis).76 Leukocytes are generally included in erythrocyte measurements, but the ratio of leukocytes to erythrocytes is usually not large enough to have appreciable effects on erythrocyte parameters (exceptions include animals with severe anemia and marked leukocytosis). In these instances the inclusion of leukocytes in erythrocyte measurements can result in increased erythrocyte counts, HCT and MCV values, and reduced MCHC values because leukocytes are larger than erythrocytes.76 These errors resulting from difficulties in separating cell type by size alone should be minimized in automated cell counters that separate cells not only by size but also by internal complexity.
With the advent of new laser flow cytometry techniques, it is now possible to perform automated differential leukocyte counts; however, most instruments cannot accurately count basophils. For most instruments, a high basophil count is an error,35 as has been noted in old blood samples.63 These flow cytometers must be specifically calibrated for each species, and they work best when leukocyte morphology is normal. More reliable flags are needed to identify the presence of left shifts and neoplastic cells.35
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree