(1)
Office of Research and Development, United States Environmental Protection Agency, Washington DC, USA
Abstract
The three diagnosis chapters—Hematology, Clinical Chemistry, and Urinalysis—are intended to be the most informative and most used parts of this handbook. There is a lot of useful information packed into these chapters. Once animal clinical pathology data have been evaluated and anomalous values for several parameters have been identified, these chapters can help one ascertain what these anomalies signify. Next to the name of each parameter are its common abbreviations. For each parameter, there are listings for organs that may be affected, specimen handling information, and supportive tests that may be used to confirm a diagnosis. These are followed by a brief description of the parameter including its strengths and weaknesses and other need-to-know information. Next to up and down arrows are potential diagnoses for when a parameter’s value is increased or decreased. When there is a name for an increase or decrease (e.g., polycythemia or anemia), that is also provided. The Hematology Diagnosis section is broken up into four sections—Erythrocytes, Leukocytes, Hemostasis, and Bone Marrow—for a total of 23 hematology parameters.
The general format used in this chapter (Hematology Diagnosis) and Chaps. 6 (Clinical Chemistry Diagnosis) and 7 (Urinalysis Diagnosis) is as follows:
5.1 Parameter Name [Abbreviations]
Affected Organs: Organs or tissues that may be affected, e.g., liver, kidneys, etc.
Specimen Handling: Information on the type of sample needed, preservation, and storage.
Supportive Tests: Other parameters that may be useful for supporting or clarifying a diagnosis.
A brief description is provided for each parameter, including its strengths and limitations.
↑ | [Name of the condition when a value is elevated, e.g., Hyperglycemia] |
Listing of potential causes for an elevated value including diseases and toxicities as well as drug and chemical interferences | |
↓ | [Name of the condition when a value is decreased, e.g., Hypoglycemia] |
Listing of potential causes for a decreased value including diseases and toxicities as well as drug and chemical interferences. ‘Not clinically significant’ means a decrease does not occur or is irrelevant |
Hematology diagnostic information is presented in the following order:
Erythrocytes
Erythrocytes (red blood cells, red blood cell count, red corpuscles, RBC)
Erythrocyte Morphology (red blood cell morphology)
Hematocrit (packed cell volume)
Hemoglobin
Mean Corpuscular Hemoglobin
Mean Corpuscular Hemoglobin Concentration
Mean Corpuscular Volume
Nucleated Erythrocytes (metarubricytes, normoblasts)
Reticulocytes (polychromatic erythrocytes)
Leukocytes
Leukocytes, Total (white blood cells, white blood cell count, white corpuscles, WBC)
Differential Leukocyte Count
Basophils
Eosinophils
Lymphocytes
Monocytes
Neutrophils (Mature or Segmented Neutrophils/Granulocytes)
Neutrophils, Band (Immature or Stab Neutrophils/Granulocytes)
Neutrophil: Lymphocyte Ratio
Hemostasis
Activated Partial Thromboplastin Time
Bleeding Time
Prothrombin Time
Thrombocytes (platelets)
Bone Marrow
Myeloid: Erythroid Ratio
5.2 Erythrocytes
5.2.1 Erythrocytes [RBC]
Affected Organs: Marrow, G.I., kidneys.
Specimen Handling: Whole blood (preserved with EDTA) may be stored 24 h at room temperature, 48 h at 4 °C.
Supportive Tests: Complete blood count (CBC), M:E ratio, serum iron, total iron binding capacity, serum ferritin, iron-binding protein, marrow iron stores, fecal occult blood.
Erythrocytes, also known as red blood cells or red corpuscles, contain hemoglobin and transport oxygen to the body. Erythrocytes are nucleated in all vertebrates except mammals. A mammalian erythrocyte is a mature cell (i.e. not nucleated) unless specified otherwise.
The erythrocyte count is a measure of the number of circulating erythrocytes per unit volume of blood (× 1012 cells/L in SI units or × 10−6 cells/mm3 in non SI units). Erythrocyte counts, hematocrit, and hemoglobin values generally rise and fall together. Excitement caused by venipuncture and exercise can cause these parameters to rise significantly due to splenic contraction. High altitude can also cause them to increase. These values fluctuate widely in young animals, and they are naturally high in ferrets (especially in males). The erythrocyte count is performed by electronic counter (the counter must be calibrated for the erythrocyte size of the species), or microscopically by the hemocytometer method.
Anemia is a significant decrease in the erythrocyte count, hematocrit, hemoglobin concentration, and/or quantity of oxygen carrying hemoglobin due to blood loss or impaired blood production (secondary to a disease state). Anemias are classified according to erythrocyte size (based on MCV values), and hemoglobin content (based on the MCHC values) as described in Chap. 2.
The opposite of anemia—erythrocytosis or polycythemia—is an increase in erythrocyte mass in the blood. Both terms are used interchangeably. The most common causes for erythrocytosis in laboratory studies are stress-induced splenic contraction, as can occur during bleeding, or hemoconcentration (relative erythrocytosis) when an animal is deprived of water. Erythrocytosis may have primary or secondary causes as described in Chap. 2.
↑ | [Polycythemia, Erythrocytosis] |
Overproduction of RBCs is usually caused by erythropoietin (EPO) stimulation following tissue hypoxia, or overcompensation following marrow suppression | |
Dehydration, anoxemia (e.g. high altitudes), methemoglobinemia, splenic contraction in response to stress or excitement (e.g., handling) | |
Polycythemia vera, congenital heart disease, bone marrow hyperplasia, renal carcinoma with increased EPO production, enlargement of liver and spleen | |
↓ | [Anemia, Erythropenia, Erythrocytopenia] |
Usually caused by aplastic anemia, marrow suppression, or loss of blood such as bleeding into the gastrointestinal tract (ulcer, colon cancer) or genitourinary tract | |
Deficiency in iron, copper, vitamin B, or protein needed for erythrogenesis | |
Coagulopathy (such as vitamin K or clotting factor deficiency), thrombocytopenia, or von Willebrand disease (in dogs) | |
Pregnancy, prolonged menstruation, abortion | |
Malaria, arboviruses in primates, gut parasitism (e.g. coccidia and hookworm) | |
Feline infectious anemia (E. felis and H. felis) | |
Malignant lymphoma (primates) | |
Hydremia (e.g. administration of intravenous fluids) | |
Anesthetics (splenic storage), surgery | |
Warfarin, arsenicals, estrogens, phenylbutazone, aspirin, sulphonamides, antihistamines, chloramphenicol, coal-tar derivatives, bracken fern poisoning, trichloroethylene-extracted feeds |
5.2.2 Erythrocyte Morphology
Affected Organs: Marrow, G.I., kidneys.
Specimen Handling: Whole blood (preserved with EDTA) may be stored 24 h at room temperature, 48 h at 4 °C.
Supportive Tests: Complete Blood Count (CBC), M:E ratio.
Wet mounts are preferable to dry blood smears for evaluating erythrocyte morphology. Blood smears are prone to artifacts caused by drying, mechanical damage, and contact with glass surfaces. Some artifacts resemble abnormal erythrocytes. Dry erythrocytes have smaller diameters.
RBCs can vary widely in size, shape, volume, staining characteristics, fragility, and hemoglobin distribution. A normal RBC is a Normocyte. Any abnormal RBC is a Poikilocyte. While poikilocytes are often associated with abnormal erythrogenesis, circulatory trauma, specific diseases, toxicity, or blood smear artifact, they may be normal in some species. Table 5.1 illustrates and describes the morphology of the most commonly observed poikilocytes. Below Table 5.1 are additional poikilocyte terms with brief descriptions. All poikilocytes are further described in the hematology glossary (Chap. 8).
Table 5.1
Erythrocyte morphology
 | Acanthocytes have projections of variable length that are unevenly spaced on the surface of the red cell. Acanthocytes may be seen as an incidental finding, as a consequence of a high-fat diet, with disorders of lipid metabolism, and with hemangiosarcoma. In the latter case, acanthocytes may form when red cells stagnate in cavernous spaces within the tumor, resulting in shifts in lipids in the RBC membrane |
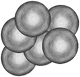 | Agglutination is identified when red cells clump or cluster together in groups (not in rows) like a bunch of grapes. Agglutination must be differentiated from rouleaux. Polychromatophils do not participate in rouleaux formation but may agglutinate |
 | Anisocytosis indicates variable red cell size |
 | Blister cells appear as though they have a hole (s) punched through the periphery of the red cell. They are observed most often in feline blood films. Blister cells may result from oxidative injury |
 | Codocytes (target cells) have a dark central area of hemoglobin, surrounded by a pale zone that in turn is surrounded by a peripheral rim of hemoglobin. Up to 50 % of canine red cells may be codocytes; they are rarely observed in other species. Increased numbers of codocytes may be present with hepatic disease |
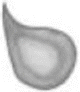 | Dacryocytes are red cells shaped like tear drops. They are considered artifactual if all points are oriented in the same direction. This artifact may be due to poor blood film preparation or lipemia. Increased numbers of non-artifactual dacryocyes may be seen with myelofibrosis |
 | Eccentrocytes have eccentric hemoglobin distribution due to annealing of a crescent of red cell membrane that excludes hemoglobin. The hemoglobinated portion of the eccentrocyte stains darkly due to a higher concentration of hemoglobin in that portion of the cell. They indicate oxidative damage to the RBC membrane and may be accompanied by RBCs with Heinz bodies |
 | Echinocytes are thought to be formed either as a result of erythrocyte dehydration or by expansion of the outer leaflet of the red cell membrane Echinocytes I are red cells with an angular shape or short, blunt projections. They are often due to artifact, such as occurs with sample aging prior to smear preparation or excessive EDTA exposure |
 | Echinocytes III are spherical red cells with sharp projections of equal length that are evenly spaced on the surface of the red cell. They may be increased in animals with renal disease and/or electrolyte disturbances. They can also occur artifactually for similar reasons described for echinocytes I |
 | Echinoelliptocytes are oval to cigar-shaped red cells with projections of equal length that are evenly spaced on the surface of the red cell. They may be seen in cats with hepatobiliary disease and are rare in other species |
 | Elliptocytes are oval to cigar-shaped cells. Red cells from Camelidae are normally elliptical |
 | Ghost cells are red cells that have been leached of hemoglobin. They are evidence of intravascular hemolysis |
 | Hypochromasia refers to red cell pallor due to inadequate synthesis of hemoglobin. Hypochromic red cells have a large area of central pallor that gradually darkens towards the periphery of the red cell. Immature RBCs may appear hypochromic due to their large size. Small (microcytic), hypochromic RBCs can be seen with iron deficiency and disorders of iron utilization |
 | Keratocytes are crescent-shaped cells. They are formed from mechanical shearing (usually due to fibrin strand deposition) of the red cell. Keratocytes are often accompanied by schizocytes (fragments) |
 | Leptocytes are thin, macrocytic red cells with a membrane surface area that exceeds hemoglobin content. The membrane tends to wrinkle or fold, forming twisted (like figure 8) cells. They are sometimes seen with hepatic disease |
 | Macrocytes (left) are larger than normal red cells |
 | Microcytes (right) are smaller than normal red cells |
 | Polychromasia refers to red cells that appear blue-gray with Romanowsky dyes. They correspond to reticulocytes on blood films stained with supravital dyes (e.g., new methylene blue, NMB). Polychromatophils are young cells with a high RNA content and, as such, are larger than mature red cells and have a different staining character. Increased numbers indicate red cell regeneration |
 | Reticulocytes can be identified on blood films stained with supravital dyes. NMB precipitates nucleic acids (like RNA) as dark blue deposits. Increased numbers indicate red cell regeneration. They correspond to polychromatophils on Romanowsky-stained smears |
 | Rouleaux are stacks of red cells. Equine and feline erythrocytes readily form rouleaux. Excessive rouleaux formation in any species may be associated with hyperproteinemia |
 | Schizocytes are red cells fragments attributed to mechanical red cell injury/shearing (see karatocytes) |
 | Spherocytes (left) are small, dark round RBCs that are formed by the removal of altered red cell membrane without concurrent loss of hemoglobin. Spherocytes have no central pallor. They may be seen with immune-mediated hemolytic anemia |
 | Unclassified poikilocytosis is used when red cell shape defies description. This term may be used to describe the peculiar (and often abundant) poikilocytosis seen in normal calves, deer, goats, and pigs, which may actually be an in vitro artifact |
 | Basophilic stippling refers to diffuse blue speckling (with Romanowsky stains) within red cells. This basophila is due to the presence of cytoplasmic RNA and reflects red cell immaturity. Increased numbers of red cells with basophilic stippling often accompany other features of red cell regeneration (especially in ruminants) such as polychromasia and reticulocytosis. Lead poisoning interferes with metabolic pathways in developing erythrocytes and may result in the presence of RBCs with basophilic stippling and metarubricytes in the peripheral blood when there is no anemia or only mild anemia |
 | Heinz bodies are difficult to visualize with Romanowsky stains where they may be visible as eccentrically located refractile bodies or blebs on the periphery of the red cell. They are better visualized and quantified on blood films stained with NMB, where they stain greenish blue. They indicate oxidative damage to red cells and may be seen along with eccentrocytes. Small Heinz bodies may be seen in high numbers on blood films from normal, non-anemic cats |
 | Nuclear remnants are small, round, dark purple erythrocyte inclusions representing a portion of the otherwise extruded nucleus. They are usually single and located close to the periphery of the red cell. Excessive numbers may be seen post-splenectomy or with hypofunctioning of the spleen |
 | Nucleated red blood cells (NRBCs) are enumerated per 100 leukocytes. Greater than 5 NRBCs/100 WBCs is significant and may indicate bone marrow damage or hypoxia. NRBCs may accompany a regenerative response when anemia is present, but should not be used as the only criterion of RBC regeneration. The total leukocyte count should be corrected if there are ≥5NRBs/100 WBCs |
Additional Poikilocytes | |
Achromocyte—ghost cell | Normocyte—normal cell |
Acuminocyte—fusiform | Ovalocyte—oval shape; elliptocyte |
Burr cell—echinocyte | Pappenheimer bodies—iron granules |
Cabot’s rings—loops & figure 8’s | Poikilocyte—any unusual RBC shape |
Crenation—notched appearance (artifact) | Punched-out cell—torocyte |
Cryohydrocyte—cold lysing | Pyknocyte—small, distorted, spiculed |
Descicyte—xerocyte | Pyropoikilocyte—distorts on heating |
Discocyte—normal cell shape | Schistocyte—schizocyte |
Drepanocyte—sickle-shaped | Selenocyte—pale, crescent-shaped |
Fusocyte—spindle-shaped | Sickle cell—drepanocyte |
Gigantocyte—extremely large | Siderocyte—iron granules |
Helmet cell—schistocyte | Spur cell—acanthocyte |
Howell-Jolly bodies—nuclear material | Stomatocyte—mouth-like slit |
Hydrocyte—swollen | Stomatospherocyte—nearly spherical |
Knizocyte—triconcave | Target cell—codocyte |
Megalocyte—unusually large | Tear-shaped—dacryocyte |
Metarubricyte—immature RBC | Torocyte—donut-shaped |
Microspherocyte—small, spherical | Xerocyte—flattened, dehydrated |
5.2.3 Hematocrit [HCT, Hct, Ht, PCV]
Affected Organs: Marrow.
Specimen Handling: Whole blood (preserved with EDTA) may be stored 6 h at room temperature, 48 h at 4 °C.
Supportive Tests: Complete blood count (CBC), M:E ratio, serum iron, total iron binding capacity, serum ferritin, iron-binding protein, marrow iron stores, fecal occult blood.
The hematocrit (also called packed cell volume, PCV) is the percentage of blood volume occupied by erythrocytes after the blood is centrifuged for approximately 5 min (10–20 min for sheep and goat blood because of small cell size). Hematocrit can be calculated by an electronic counter from the RBC count and MCV provided the counter has been calibrated for the erythrocyte size of the species. The microhematocrit method does not require expensive equipment, but it is prone to error when centrifugation fails to remove trapped plasma. The buffy coat layer of platelets and leukocytes is not included in the hematocrit measurement.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


