(1)
Office of Research and Development, United States Environmental Protection Agency, Washington DC, USA
Abstract
The Hematology Highlights chapter begins with a description of hematopoiesis—the process by which all blood cell species are formed—that takes place primarily in the bone marrow but also in the liver, and spleen. A section on erythrocytes (RBCs) examines the causes of anemia, the morphologic classifications of anemias, the process whereby healthy bone marrow responds to anemia, the causes of erythrocytosis, and 24 major causes of hemolysis. The section on leukocytes (WBCs) describes the formation of neutrophils, lymphocytes, eosinophils, monocytes, and basophils and the role each plays in the immune system. A discussion of differential leukocyte counts describes how each of the leukocyte species can be presented as either percentages of WBCs or as absolute cell counts. An example illustrates why evaluating differential cell counts only as percentages can often lead to a misdiagnosis. Finally, the formation of thrombocytes (platelets) and the key role they play in hemostasis are described.
2.1 Hematopoiesis
In a process called hematopoiesis, all blood cell types are produced through pathways that begin with multipotent stem cells called hemocytoblasts and hemoblasts. Figure 2.1 illustrates the hematopoietic pathways for each blood cell type. Each cell lineage undergoes a series of proliferative phases (mitotic division) and maturation phases, after which they enter a storage pool of mature cells for release into general circulation and, in the case of some leukocytes, distribution to the body. All blood cell types except thrombocytes undergo a series of mitotic divisions followed by several maturation stages during which they shrink in size. Thrombocyte production is unique in that the marrow precursor cells undergo polyploidy without cell division. This results in very large cells with multilobed nuclei that then break up into hundreds of platelets. Hematopoiesis primarily occurs in the bone marrow but it can also occur in the spleen and liver, especially in laboratory mice and rats. The rodent spleen produces mostly erythrocytes but it can also produce granulocytes and megakaryocytes.
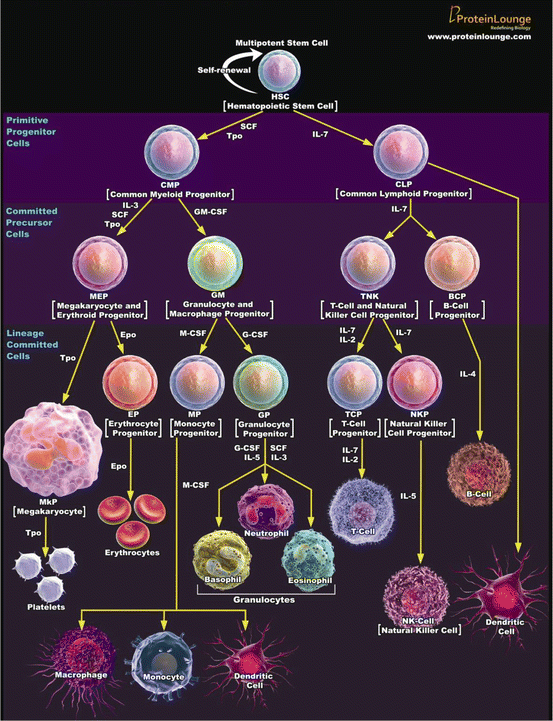

Fig. 2.1
Hematopoiesis from multipotent stem cell
Flow cytometry (FCM) is the preferred method for counting RBCs, WBCs, reticulocytes, and platelets. Reticulocyte counts can also be made by counting ≥1,000 erythrocytes in a smear stained with new methylene blue. Hemoglobin is measured by the cyanmethemoglobin method. Wright-stained blood smears are used to microscopically evaluate erythrocyte, leukocyte, and platelet abnormalities, leukocyte counts, differential leukocyte counts, parasites, platelet counts, and bacteria. Wet mounts are preferred for morphology studies since erythrocyte shape is unaltered, and artifacts are minimized. Erythrocyte morphology is further described in the Hematology Diagnosis chapter and the Hematology Glossary. An increase in a cell count has the suffix—philia or—osis (e.g. neutrophilia, monocytosis). A decrease in a cell count has the suffix—penia (e.g. lymphopenia).
Figure 2.1 illustrates the hematopoietic pathways by which various blood cells are produced from multipotent stem cells. Each step of this process is regulated by the cytokines and growth factors adjacent to the yellow arrows. [This illustration was created by proteinlounge.com and is used with their kind permission.]
2.2 Erythrocytes
Approximately one million senescent erythrocytes are replaced every second in the dog. In response to anemia, such as from blood loss or disease, a healthy dog’s bone marrow can produce erythrocytes at 6–8 times the normal rate. The factors needed for erythrocyte production are erythropoietin (EPO, the key cytokine for regulating RBC production), globulins, iron, cobalt, copper, hematopoietic factor, protoporphyrin, vitamins B2, B6, and B12, niacin, folic acid, and thiamine. The lack of any of these can lead to anemia, polycythemia, and abnormal erythrocytes.
Anemia is a decrease in the erythrocyte count, hematocrit, and/or hemoglobin. Anemia is not a disease, but rather a sign of disease or trauma. There are four general causes of anemia:
Blood loss—trauma, surgery, coagulopathies, blood-sucking parasites, intestinal or genitourinary bleeding, internal hemorrhage, ulceration, and poisoning (warfarin, bracken fern, sweet clover).
Hemolytic anemias—the bursting of RBCs caused by bacterial and viral infections, chemicals (e.g. lead, saponin, copper, and certain drugs such as phenothiazine), poisonous plants, metabolic diseases, and hemolytic diseases.
Bone marrow depression—physical agents (radiation), chemicals (bracken fern, estrogen, phenylbutazone, some antibiotics), parasites, infectious agents, chronic infections, nephritis, liver disease, endocrine deficiencies, and myeloproliferative disease.
Nutritional deficiencies—vitamin, mineral, and protein deficiencies.
Anemias are either regenerative or non-regenerative (sometimes called responsive or non-responsive anemias). In regenerative anemias, the bone marrow increases erythrocyte production to replace lost or hemolyzed cells, and releases large (macrocytic) immature RBCs such as nucleated erythrocytes and reticulocytes. This response is not seen in non-regenerative anemias.
Table 2.1 shows how anemias can be morphologically classified using two erythrocyte indexes—mean corpuscular volume (MCV) and mean corpuscular hemoglobin concentration (MCHC). Typically, macrocytic anemias are regenerative (responding), normocytic anemias are non-regenerative (non-responding), and microcytic anemias may be either.
Table 2.1
Morphologic classification of anemias
MCV | MCHC | Interpretations |
|---|---|---|
Normocytica | Normochromic | Normal Depression anemias (excluding certain nutritional deficiencies and some cases of myeloproliferative disorders in the cat) |
Normocytica | Hypochromic ↓ | Early iron deficiency, depressed erythrogenesis, disease in domestic animals |
Macrocyticb ↑ | Normochromic | Pernicious anemia in primates Vitamin B12 and folate deficiencies Cobalt deficiency in ruminants Erythemic myelosis and erythroleukemia in cats Defective erythrogenesis as in Poodle macrocytosis Antimitotic drugs, liver disease, splenectomy |
Macrocyticb ↑ | Hypochromic ↓ | Temporary anemia following blood loss or hemolysis, usually accompanied by reticulocytosis |
Microcyticc ↓ | Normochromic | Normal for Japanese Akita dogs. Iron deficiency in progression |
Microcyticc ↓ | Hypochromic ↓ | Iron and copper deficiencies and chronic blood loss. Pyridoxine (vitamin B6) deficiency |
The opposite of anemia—erythrocytosis or polycythemia—is an increase in erythrocyte mass in the blood. Both terms are used interchangeably. The most common causes for erythrocytosis in laboratory studies are stress-induced splenic contraction or hemoconcentration (relative erythrocytosis) when an animal is deprived of water. Erythrocytosis may have primary or secondary causes:
In primary erythrocytosis, there is splenomegaly, a dramatic increase in erythrocyte production despite low serum EPO levels, and increased production of granulocytes and thrombocytes. Primary erythrocytosis is a myeloproliferative disease. It is also called primary polycythemia, polycythemia vera, and polycythemia rubra vera.
In secondary erythrocytosis, there is an increase in the erythrocyte count in the circulating blood because of excessive EPO production, either due to systemic hypoxia (appropriate) or an EPO secreting tumor in the kidney (inappropriate). Unlike primary erythrocytosis, there is no increased production of granulocytes and thrombocytes. It is also called Secondary Polycythemia.
A common finding in toxicity studies is hemolysis, the lysing (bursting) of erythrocytes with the release of hemoglobin. Hemolysis occurs naturally in the spleen, liver, and lymph nodes, which each have abundant macrophages. Hemolysis in peripheral blood can be caused by many conditions, diseases, toxicities, pharmaceuticals, and sampling errors, as demonstrated in Table 2.2.
Table 2.2
Major causes of hemolysis
• Inherited conditions (e.g., thalassemia, hematoglobinopathy, sickle cell anemia) | • Antiviral agents (e.g., Ribavirin®) |
• Metabolic diseases | • Hemodialysis |
• Hemolytic diseases | • Transfusion reactions |
• Autoimmune hemolytic anemia | • Malaria |
• Poikilocytes (abnormally shaped RBCs) | • Tick-borne diseases |
• Improper specimen collection (improper venipuncture, use of a small or large-bore needle, rapid transfer of blood from a syringe to a tube) | • RBC parasites (e.g., babesiosis) |
• Improper specimen processing (vigorous mixing, exposure to heat or cold) | • Arsenic |
• Bacterial and viral infections (e.g., clostridial organisms) | • Poisonous plants |
• Pharmaceuticals (e.g., sulfones, dapsone, quinine, nitrofurantoin, sulfonamides, phenazopyridine phenothiazine) | • Metals (chromium/chromates, platinum salts, cis-platinum, nickel compounds, copper, lead)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|