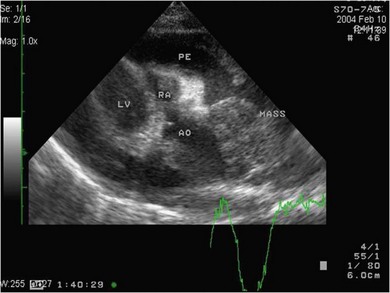
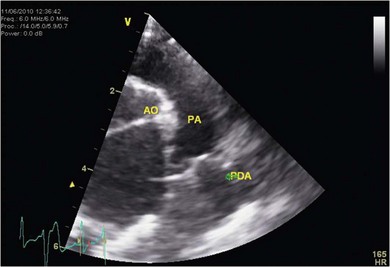
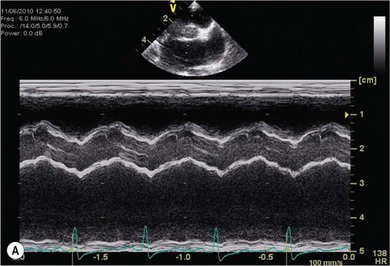
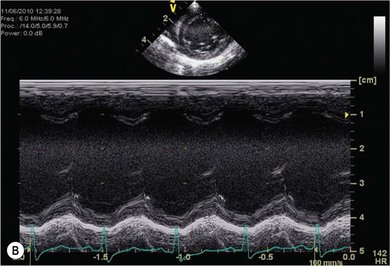
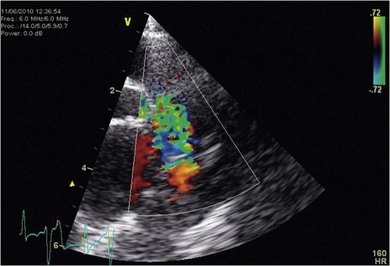
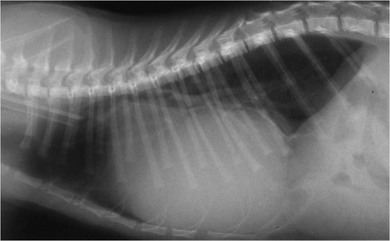
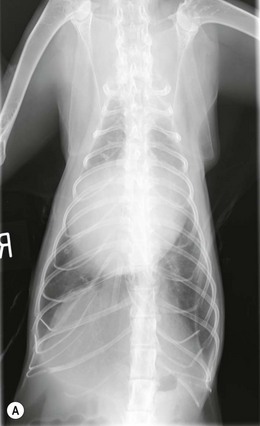
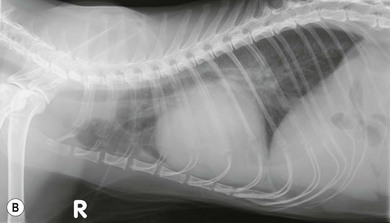
Chapter 48 There is little variability in normal thoracic conformation and heart shape between cat breeds. The normal feline heart is slender in appearance on a lateral thoracic radiograph and it appears more horizontal in its orientation within the thorax than in the dog; with increasing age this can become more marked.1 The heart lies within the mediastinum in the mid thorax, approximately between the 3rd and 6th intercostal spaces. The ductus arteriosus carries blood from the pulmonary artery to the aorta in the fetus. Within hours of the birth the ductus normally closes in response to an increase in the oxygen tension of the blood; failure of closure allows continual shunting of blood between the left and right sides of the heart. After birth, the blood pressure in the pulmonary artery reduces and the systemic blood pressure increases. Typically this pressure differential is such that blood shunts continuously through the cardiac cycle, from the higher pressure aorta to the lower pressure pulmonary artery (i.e., left to right shunting) if the ductus remains patent. The secondary changes that occur with increased blood flowing in the pulmonary artery are pulmonary over-circulation, left atrial dilatation, left ventricular dilatation with eccentric hypertrophy, dilatation of the aortic arch to the level of the origin of the PDA, and dilatation of the pulmonary trunk. As a consequence of the left to right shunting and left-sided volume overload, left-sided congestive heart failure (CHF) commonly develops. The size of the shunt will determine volume of blood shunting, which in turn determines the onset and severity of secondary consequences. As a sequel to the increased blood flow in the pulmonary vessels, resistance to blood flow and pulmonary hypertension can develop. If the pulmonary arterial pressure is greater than the aortic pressure for some or all of the cardiac cycle, blood flow through the PDA will reverse. Blood will then flow from the pulmonary artery into the aorta (i.e., right to left shunting). Right to left shunting can also occur from birth, and usually occurs when the ductus is large and tubular without narrowing at the pulmonary ostium, and this may reflect retention of fetal pulmonary vasculature. Right to left shunting, termed Eisenmenger’s physiology, is rarely reported in cats,2,3 although it is suggested that it is more likely to develop over time in cats than dogs.4 Surgical treatment of the PDA is contraindicated in patients with right to left shunting. PDA is a rarely diagnosed congenital heart disease in the cat, with few cases of this condition reported in the English language veterinary literature so it is difficult to make conclusions about the presentation, investigative findings, and the outcome with medical or surgical treatment. This contrasts with dogs, where PDA is one of the more commonly diagnosed congenital heart diseases5,6 and it is well documented. Most dogs are diagnosed as puppies, whilst the condition is asymptomatic, by auscultation of a pathognomic continuous murmur. It is suggested that the situation may be similar in cats, and certainly the majority of cats reported with PDAs are less than one year of age. However, in an abstract reporting 21 cases of PDAs in cats approximately two-thirds had a continuous murmur but the remainder were described to have systolic murmurs and approximately only one-third of the cats in the study were asymptomatic at presentation. Their presenting complaints included abnormal respiration, exercise intolerance/lethargy, stunted growth, and poor weight gain.7 The murmur is well described in dogs; it may be accompanied by a thrill felt at the heart base, and by hyperkinetic pulses. The point of maximal intensity of the murmur lies over the main pulmonary artery at the dorsocranial heart base and may radiate cranially to the thoracic inlet and to the right heart base. It is suggested that in cats the continuous murmur of a PDA may be best heard slightly more ventrally than this typical location in dogs. Femoral pulses may be more difficult to assess in cats;8 hyperkinetic pulses may be noted. Diagnosis is usually confirmed with echocardiography using a right parasternal short axis or left parasternal cranial window (Fig. 48-1). Initially, there may be no changes in chamber size or myocardial contractility but eventually the consequences of left to right shunting may result in dilatation of the left atrium, aorta, and main pulmonary artery and dilatation and hypertrophy of the left ventricle, all recognizable on echocardiography (Fig. 48-2). Left ventricular systolic function may become impaired. If a left-to-right shunting PDA is identified, Doppler studies can be performed and will show characteristic high velocity turbulent flow from the aorta, through the ductus and into the main pulmonary artery (Fig. 48-3). This PDA peak velocity will decrease if pulmonary pressures increase, prior to exceeding systemic pressures. However, note that this decrease may be reactive pulmonary hypertension as a consequence of the pulmonary over-circulation and closure of the PDA may resolve the mild pulmonary hypertension when assessed after surgery. Mild increase of velocity in the left ventricular outflow tract and mild aortic and pulmonic insufficiency may also be recognized. Mitral regurgitation is secondary to the left-sided dilation and may be significant. Figure 48-1 Right parasternal short axis view of the heart in a cat with a patent ductus arteriosus (PDA). This image was taken at the level of the pulmonary artery and shows the PDA. (Courtesy Dr Jo Dukes-McEwan.) Figure 48-2 A cat with a left-to-right shunting patent ductus arteriosus (PDA). (A) Aortic M-mode image showing marked left atrial dilatation relative to the size of the aorta. (B) M-mode image of the left ventricle showing left ventricular dilatation, relative wall thinning and impaired systolic function. (Courtesy Dr Jo Dukes-McEwan.) Figure 48-3 Right parasternal short axis view of the heart in a cat with a patent ductus arteriosus (PDA). This image was taken at the level of the pulmonary artery with color flow Doppler showing turbulent flow across the PDA. (Courtesy Dr Jo Dukes-McEwan.) Thorough echocardiography should be performed in cats because concurrent, but unspecified, congenital heart defects may be present; these were found in 29% of cats in one study.7 Congenital pericardial diseases are infrequently diagnosed and most have an excellent prognosis with surgical treatment. The most common condition in dogs and cats is peritoneopericardial diaphragmatic hernia (PPDH);9 it is more common in cats (see Chapter 45). Intrapericardial ‘cysts’ are rarely reported.10–14 These are usually not true cysts but encapsulated adipose tissue or organizing cystic hematomas. The cyst may be attached to the apex of the pericardium by a pedicle. They may arise if a PPDH containing herniated omentum or falciform fat closes before birth, trapping the fat within the pericardial sac. Cystic changes of herniated and incarcerated liver tissue may also occur in association with PPDH in cats. Intrapericardial cysts cause clinical signs either directly associated with their presence, or due to an associated effusion causing cardiac tamponade. Diagnosis can usually be made by radiography (Fig. 48-4) and/or ultrasound examination of the cranial abdomen and heart. Treatment is by subtotal pericardectomy (see Box 48-4), removal of the cyst (and pedicle if present), and repair of the PPDH (Chapter 45, 45.4.3) if present. Figure 48-4 Lateral thoracic radiograph of a young, anesthetised cat with a peritoneopericardial diaphragmatic hernia (PPDH). The cardiac silhouette is markedly enlarged and silhouettes with the diaphragm. The distal intrathoracic trachea is displaced dorsally over the cranial border of the cardiac silhouette. PE in cats is most commonly part of a more generalized disease (Box 48-1). Myocardial disease is the commonest underlying cause.15 Small volume clinically insignificant PEs have been reported in approximately 45% of cats with CHF,16,17 while PEs are rarely reported in dogs with CHF. The other most frequent underlying cause of PE in cats is feline infectious peritonitis. The commonest causes of PE in dogs are idiopathic hemorrhagic pericardial effusion (IPHE), which is unreported in the cat, and cardiac-related neoplasia. In dogs, hemangiosarcoma, usually of the right side of the heart, is the most common neoplastic cause of PE, aortic body tumors also occur with some frequency, mesotheliomas occur less commonly, and other tumors are also reported. These tumors are rarely diagnosed as a cause of PE in cats; lymphoma is the most common underlying tumor associated with the effusion. The classic radiographic appearance of PE is general cardiomegaly (globoid heart) and sharp borders to the cardiac silhouette (Fig. 48-5). If the PE has accumulated rapidly without time for adaptation, cardiac tamponade may occur without the classic radiographic signs being present. Pleural effusion is usually present in cats with significant PE and may partly obscure the cardiac silhouette. Figure 48-5 (A) Dorsoventral and (B) lateral thoracic radiographs of a cat with a pericardial effusion. There is a small volume of pleural fluid. The underlying heart disease in this cat was severe hypertrophic cardiomyopathy. (Courtesy Dr Jo Dukes-McEwan.) A definitive diagnosis of PE is made by echocardiography, which allows cardiac disease or mass lesions to be excluded. The pericardial fluid is seen as an anechoic/slightly anechoic circular region surrounding the heart, within the thin hyperechoic line representing the pericardium. Although an uncommon cause of PE in cats, mass lesions affecting the heart may be seen on ultrasound examination. A thorough examination of the heart should be performed prior to pericardiocentesis because masses are most likely to be identified whilst surrounded by fluid (Fig. 48-6).
Heart
Surgical anatomy
Surgical diseases
Patent ductus arteriosus
Diagnosis
Pericardial disease
Intrapericardial cysts
Pericardial effusion
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Heart