, Monika Hassel2 and Maura Grealy3
(1)
Centre of Organismal Studies, University of Heidelberg, Heidelberg, Germany
(2)
Spezielle Zoologie, Universität Marburg FB Biologie, Marburg, Germany
(3)
Pharmacology and Therapeutics, National University of Ireland Galway, Galway, Ireland
17.1 From Seemingly Chaotic Origins to High Order
17.1.1 Heart and Vessels Arise in Many Separate Locations But Unite to Form One System in the End
The heart is the first organ to form and the cardiovascular system is the first functional system in the developing embryo. Both the heart and the blood vessels develop from the mesoderm at the same time so that a complete circulatory loop is ready for the start of cardiac contraction. In humans this is at about the third week of gestation. Despite divergent origins in different vertebrates, in the medium (phylotypic) and late stage of development the vascular systems faithfully repeat evolutionarily ancient structures in a highly conservative manner. Similar to the nervous system mechanisms of self organization create highly ordered structures in a reproducible manner. The anatomical construction of the circulatory system in the model animal fish and in mammals/humans is outlined in Figs. 17.1, 17.2, 17.3, and 17.4.
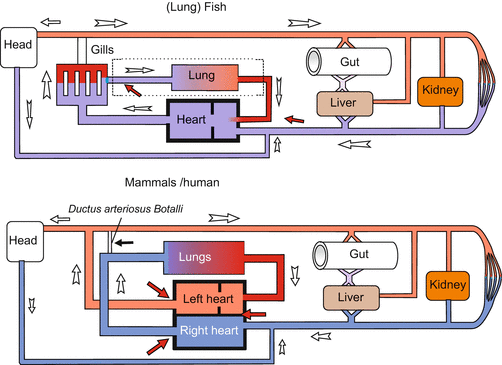
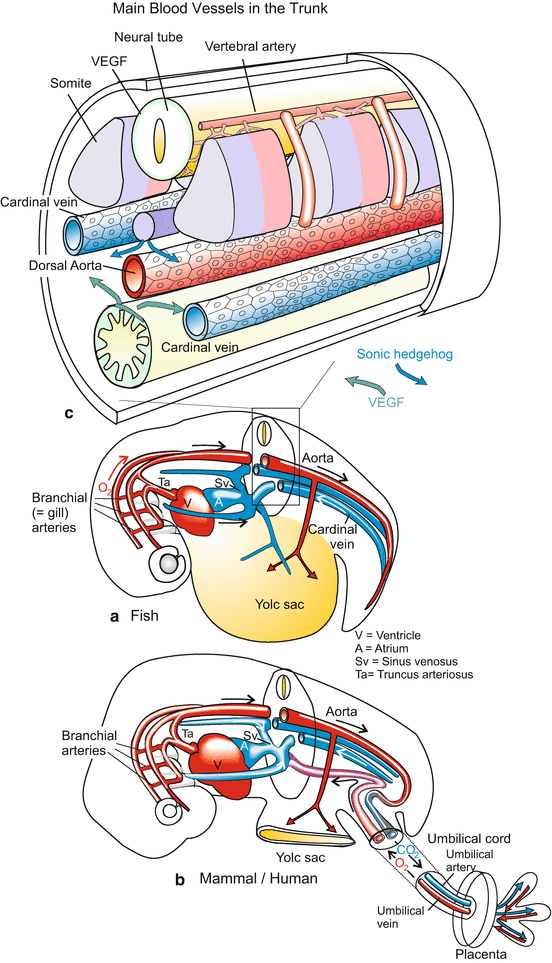
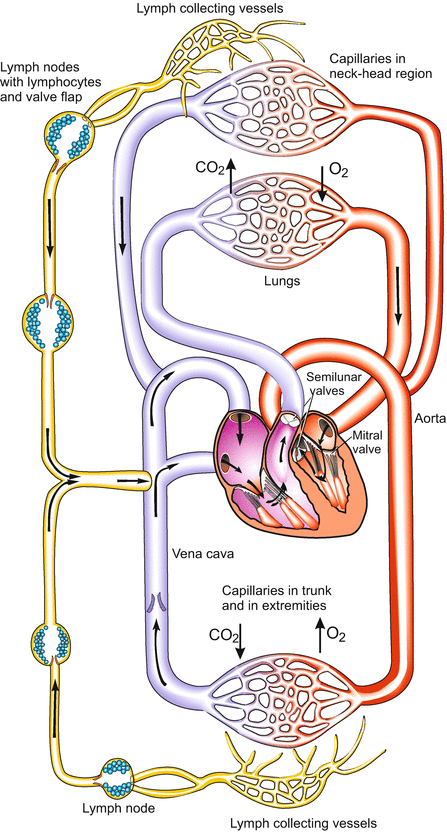
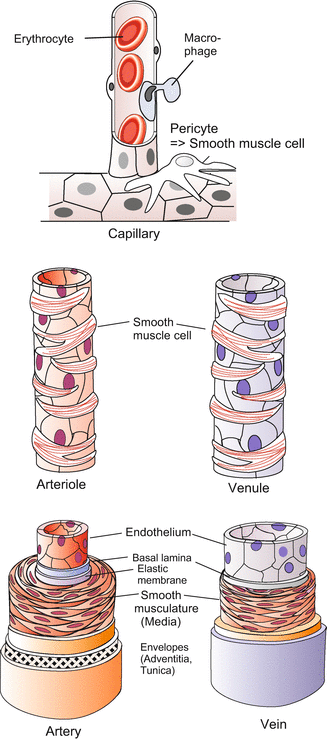

Fig. 17.1
Circulatory system in adult fishes and mammals/humans. Vessels transporting oxygenated blood are in red, vessels carrying deoxygenated blood are in blue. (In lung fishes the lung functions as an accessory respiratory organ and is supplied by blood from a side branch of the last branchial arch). From Mueller and Frings (2009)

Fig. 17.2
Embryonic circulatory system in fish (a), and mammals/humans (b). Essentially, the anatomical construction is the same. However, in hatched fish larvae oxygen enters the blood in the gills and is distributed via the dorsal aorta. In mammals oxygen enters the blood in the placental villi and is forwarded by the umbilical vein. Arteries defined as vessels leading from the heart are in red, vessels carrying blood to the heart are in blue, irrespective whether vessels transport oxygenated (“arterial”) blood or deoxygenated (“venous”) blood. The upper figure (c) shows a section of the trunk with the main vessels. The section is similar in larval fish and mammalian embryos (a, b, own figures, c after Coultas et al. 2005, modified)

Fig. 17.3
Human circulatory system after birth. From Mueller and Frings (2009)

Fig. 17.4
Histological structure of blood vessels. Mueller and Frings (2009)
To avoid misunderstandings we recall basic biological knowledge, especially as the traditional terminology can be misleading. By definition arteries are vessels leading from the heart; they are usually shown in red, while veins are vessels leading to the heart, they are usually shown in blue. In terms of physiology the arterial system is the high pressure system, the venous system is the low pressure system. This is reflected in the different structure of the walls of arteries and veins (Fig. 17.4). In connection with blood the adjective “arterial” means oxygenated: arterial blood is loaded with oxygen O2, while the adjective “venous” means deoxygenated: venous blood is unloaded from oxygen but loaded with carbon dioxide CO2. As a rule, arteries transport arterial blood, veins venous blood, but this is merely a rule and there are important exceptions. For instance, in the mammalian embryo only the vein coming from the placenta carries oxygenated “arterial” blood, and in the adult system the lung arteries carry deoxygenated, “venous” blood, while the lung veins transport oxygenated, “arterial” blood. In the context of this chapter the colours red and blue in the Figures can have a different meaning; therefore reading the legends is important.
17.1.2 Blood Vessels and Heart Are Formed by Migratory Precursor Cells
Both the branching blood vessels and the massive heart are derived from common cardiovascular precursor cells which arise from Brachyury-expressing mesoderm close to the midline on both sides of the embryo. These then differentiate into either cardiac progenitors or haemangioblasts (precursors for blood vessels and blood cells). The cardiac progenitor cells migrate anteriorly whereas the haemangioblasts migrate posteriorly (caudally). These wandering mesodermal cells have the progeny as shown in Fig. 17.5. The stem cells of the various blood cells including the cells of the immune system will be dealt with in Chap. 18 (stem cells, see Fig. 18.6).
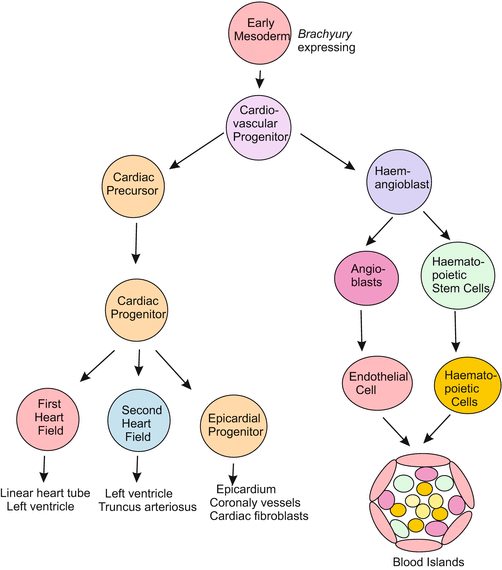

Fig. 17.5
Lineage of cells involved in the formation of the heart und circulatory system. After O’Connor et al. (2013)
17.2 The Heart
17.2.1 Aristotle’s Jumping Point, the Heart, Arises From migratory Cardiac Progenitor Cells
Aristotle, when opening incubated eggs, observed a ‘jumping point’ in the blastodisc. Even without magnifying glasses he identified the jumping point as a beating heart.
The fish and avian embryos lend themselves well to studies of heart development. The events described below occur in a similar way in other vertebrates as well (Figs. 17.6 and 17.7A,B). The circulatory system is established early in development during neurulation when diffusion is no longer sufficient to supply the embryo with nutrients and oxygen.
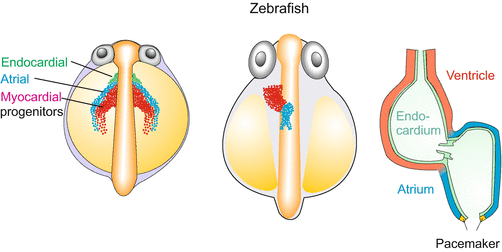
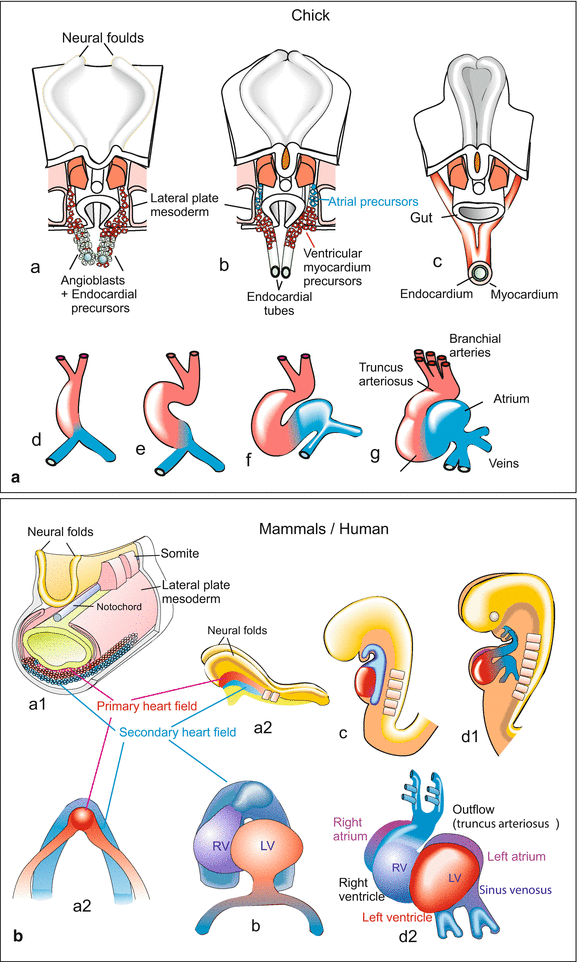

Fig. 17.6
Development of the zebrafish Danio rerio. Development before looping. Embryos are shown from a dorsal view. Three cohorts of migratory cells form the core of the heart. A first cohort (green) provides the endothelial layer, the second cohort (red) emigrates from the primary heart field and consist of myocardial progenitors which will form the ventricle. The third cohort (blue) emigrates from the second heart field and consist of myocardial cells that will form the atrium. The heart is supplemented by epicardial cells (not shown). After Staudt and Stainier (2012)

Fig. 17.7
(A, B) Development of the avian and the mammalian heart. Note that in these Figures the colours red and blue indicate the derivation of the progenitor cells from the first heart field (red) or from the second field (blue). The colours do not indicate whether the vessels will become arteries or veins by definition nor do they give hints to the future state of oxygenation of the blood. (A) The heart of a bird. The heart primordium originates from migrating angioblasts and myocardioblasts which aggregate to form paired tubes (a, b). The first descendants of the angioblasts form the inner wall of the tubes, called endocardium, the myocardioblasts form the muscular wall, the myocardium (c). The paired tubes fuse forming the unpaired looped heart tube. (d, e, f) The heart tube subdivides into the sections sinus venosus, atrium, ventricle, and truncus arteriosus (g). B Heart development in a generalized mammal (mouse or human). Also in mammals the primary and secondary heart fields provide the progenitors of the muscular myocardium. Red: Progenitors from the primary (first) heart field, blue progenitors derived from the second heart field. After several authors (e.g. Xin et al. 2013) and own figures, combined and modified
The cardiac progenitor cells migrate anteriorly on both sides of the midline and fuse to form the primary or first heart field, also known as the cardiac crescent. Cells from this first heart field involute to form the linear heart tube. This tube first elongates, then moves to the left side of the midline and loops to the right to form two chambers, the atrium and the ventricle. A second wave of cells derived from the same precursor population differentiates and migrates later; this is called the second heart field. Cells from the first heart field contribute mainly to the left ventricle, whereas those from the second heart field give rise to the right ventricle, the atria and the outflow tract. The embryonic heart comprises three layers (Fig. 17.6):
Endocardium. The origin of the endocardium is controversial, but it is now thought that both the first heart field and endothelial lineages can give rise to endocardium. The endothelial-derived cells are found adjacent and medial to the first heart field (cardiac crescent). They migrate at the same time as the second heart field and form an inner lining on the myocardial cells.
Myocardium. The power-generating myocardium of the heart, consisting of cross-striated muscle fibres, arises from precursor cells from the first and second heart fields.
Epicardium. This outermost layer of the heart is formed from a transient cell cluster called the proepicardium. This cluster of cells likely arises from the second heart field under the control of FGF8. It is first seen at the heart looping stage near the inflow tract. The cells spread over the myocardium, forming a single epithelial layer.
17.2.2 While Beating the Bending Tube Transforms Into to a Chambered Heart
Once the heart tube has formed (initially from the first heart field) it elongates by addition of cells from the second heart field at both poles. It then loops to the right to form two chambers, the atrium and the ventricle. The atrio-ventricular valve forms and cardiac electric conduction begins. In fishes a two-chambered heart is the final stage. In mammals the septum forms and further folding results in a four-chambered heart.
The functional units in the embryonic heart are: The
sinus venosus and atrium collect venous blood, forwarding it to the
ventricle, the power-generating pump; it drives the blood into the
truncus arteriosus which directs the expelled blood to the distributing branchial arteries.
This simple heart, consisting of one pump, satisfies the needs of a fish all its life. In land vertebrates the embryos also require only a single pump, since a separate circulatory system enclosing the collapsed lung is not yet necessary. But even before birth, land vertebrates – especially birds and mammals – must be prepared for a life in air.
Without missing a beat, the mammalian heart is folded and reconstructed to yield two functional pumps with two chambers each; one pump to propel the volume of blood through the body (large or systemic circulation), the other pump in anticipation of the needs after birth when the lungs must function. The process by which the folded tube is transformed into the final heart is quite complex and can be outlined here only in principle.
The double heart arises by in-growing of transversal partition walls. In amphibians and reptiles these walls remain incomplete, but in birds and mammals the chambers are completely separate (except the foramen ovale, see Sect. 17.4.2). A double pump is needed to separate oxygenated from venous blood and to overcome the high resistance in the lung capillaries. It is not sufficient, however, to separate atrium and ventricle by a partition wall. Also the venous input vessel and the arterial output vessel must be split off and the doubled vessels must be criss-crossed.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


