Chapter 47 Haemosporidian Parasites
Impacts on Avian Hosts
Haemosporidian parasites (order, Haemosporidia; phylum, Apicomplexa) are cosmopolitan intracellular protozoan parasites of birds, reptiles, and mammals.25 Haemosporidian parasites develop in two types of hosts, vertebrates and invertebrate vectors (Insecta, Diptera, blood-sucking dipterans); the dipteran is considered the definitive host because it is the site of sexual reproduction. Avian haemosporidia include parasites from three genera—Plasmodium, which is typically vectored by mosquitoes (Culicidae); Haemoproteus, which is primarily transmitted by biting midges (Ceratopogonidae) and louse flies (Hippoboscidae), and Leucocytozoon, which is vectored by blackflies (Simuliidae). Historically, Plasmodium has been considered potentially very pathogenic and Haemoproteus relatively benign. In this chapter, we will summarize studies relevant to these common perceptions and present a detailed case study of an ongoing investigation of what is thought to be a recent arrival of Plasmodium in a naïve island population.
Life Cycle of Haemosporidians
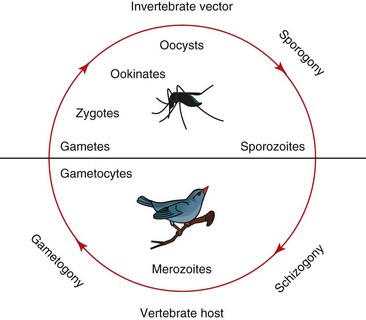
The life cycle consists of several stages in tissue and circulating blood cells of infected hosts. An infected vector feeds on vertebrate host blood, inoculating the host with sporozoites and giving rise to agamic stages (referred to as exoerythrocytic meronts or schizonts), which undergo asexual reproduction in parenchymal tissue in the host. This asexual division, often called merogony or schizogony, results in uninuclear merozoites. Another cycle of merogony occurs in the host blood cells in Plasmodium, from which the parasite proceeds into the development of gametocytes; parasites in the genus Haemoproteus move quickly into the gametocyte stage in the blood. These cells produce macrogametocytes and microgametocytes, which are infective for the vectors. When an arthropod vector feeds on an infected bird, the change in carbon dioxide and oxygen concentrations and temperature initiate gametogenesis in the midgut of the vector, resulting in a sexual process called oogamy. Macrogametocytes produce macrogametes, microgametocytes produce microgametes, and fertilization occurs extracellularly. The zygote forms an elongated mobile ookinete, which penetrates the epithelial layer of the vector’s midgut, where it develops into an oocyst. Sporozoites, the stage that is infective for the vertebrate hosts, are formed in the oocyst and later move into the haemocoele of the vector, eventually penetrating the salivary glands. From there they may complete the infection cycle when the mosquito takes a second blood meal (Fig. 47-1).
Pathogenicity
Much of our understanding of the pathogenicity of haemosporidian parasites is based on laboratory experiments on domesticated birds (canaries, chickens, ducks, pigeons, turkeys) or on accounts from infections in birds housed in zoos. In a review of pathogenicity of haemosporidian parasites in birds, Bennett and colleagues4 found that 89% of published articles (5640 total) detailed mortality in domesticated birds, whereas 6% and 5% pertained to mortality in zoo and wildlife populations, respectively.
Captive Populations
Haemosporidian parasites (primarily Plasmodium relictum and P. elongatum) cause severe morbidity and mortality in penguin populations in zoos.4 Most of the world’s penguins are distributed near polar regions, where haemosporidia are scarce. Therefore, many of the penguin species found in zoos have not evolved in regions that support populations of suitable vectors, resulting in naïve hosts, which in turn contributes to the severity of the infections. Many of the examples of mortality in zoos caused by haemosporidia involve hosts challenged by parasites not found in their native distribution. One notable example is that of four Keas (Nestor notabilis) that were captured in New Zealand and moved to the Malaysian National Zoo in 1964. Native Kea habitat in New Zealand was free of haemosporidia but, in captivity in Kuala Lumpur, where they were exposed to many blood-feeding vectors carrying local lineages of haemosporidia, all four died after 3 weeks in the new location because of infection by at least two Plasmodium species. Leucocytozoon spp. were found to be particularly pathogenic for birds in the orders Galliformes and Anseriformes (poultry and ducks, respectively).
Haemosporidian Parasites in Hawaii
Intensive, long-term laboratory and field experiments have been conducted on Hawaiian avifauna, providing us with a complete understanding of the susceptibility of extant bird species to Plasmodium, the distribution (both across host species and in different habitats or elevation), and the prevalence (proportion of individuals infected) and intensity (proportion of cells infected within an individual) of infections in birds and in vectors. Native species were more susceptible to Plasmodium than were introduced species and more likely to have microscopically detectable infections during the nonbreeding season.18 Many surviving species, particularly the susceptible and consequently endangered ones, persist only above 1500 m of elevation, where cooler temperatures prevent Plasmodium from effectively developing in mosquitoes. However, because of climate change and warmer temperatures, the prevalence of Plasmodium in Hawaiian forest birds sampled at 1900 m has more than doubled in over a decade.8 Some Hawaiian bird species appear to be coping. The Amakihi (Hemignathus virens), which exists in lowland areas in which mosquitoes and Plasmodium are prevalent, showed no significant reduction in reproductive success—as measured by clutch size, hatching success, fledging mass, number of nestlings fledged, daily survival, and minimum fledgling survival—while chronically infected with P. relictum.11 These results are consistent with the hypothesis that offspring inherit genes for Plasmodium resistance from their infected parents that lead to increased survival, so it appears that the Amakihi is now a good reservoir for the parasite in the forest bird community. It remains unknown whether resistance will evolve in other species, because this requires both a growing population of resistant birds and heritable resistance to acute Plasmodium infection.
Impact on Long-Term Associations and Comparison of Impact across Parasite Genera
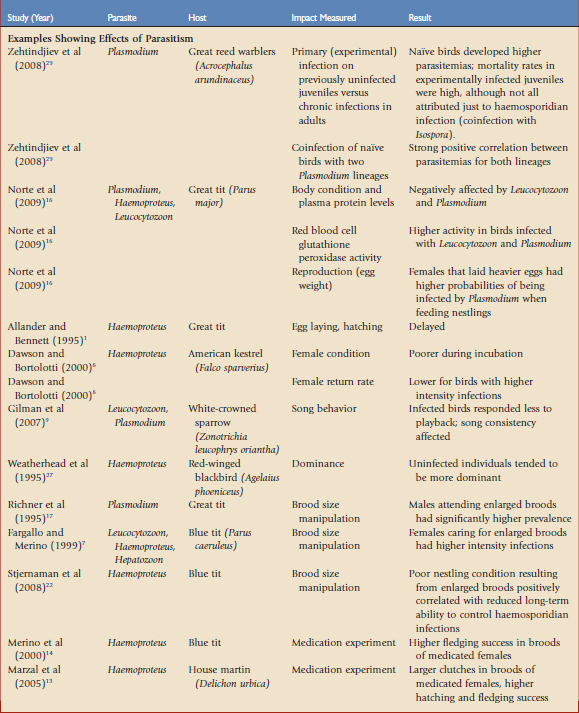
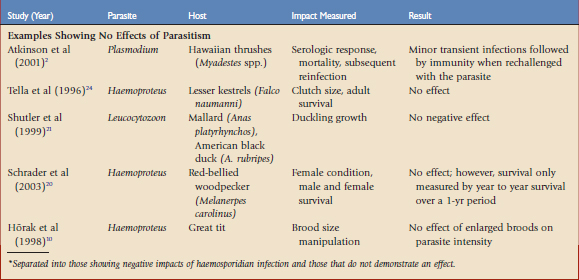
Haemosporidian parasites have been shown to affect hosts in situations in which the hosts have presumably evolved with both the vectors and parasites for far longer than in the case of the Amakihi in Hawaii. Much of the research on fitness consequences of haemosporidian has relied on correlative data in wild populations. Although these studies are important in adding to our understanding of the effects of these parasites, experimental manipulation may tease out the causal relationships involved. There are two main experimental approaches to understanding the impacts of haemosporidians on host fitness, brood size manipulation and medication experiments. By manipulating the reproductive effort or reducing natural parasite infection, experiments may reveal causal relationships. Some correlative and experimental studies that have demonstrated a potential fitness cost to haemosporidian parasites, and those that have shown no effect, are summarized in Table 47-1.19
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree