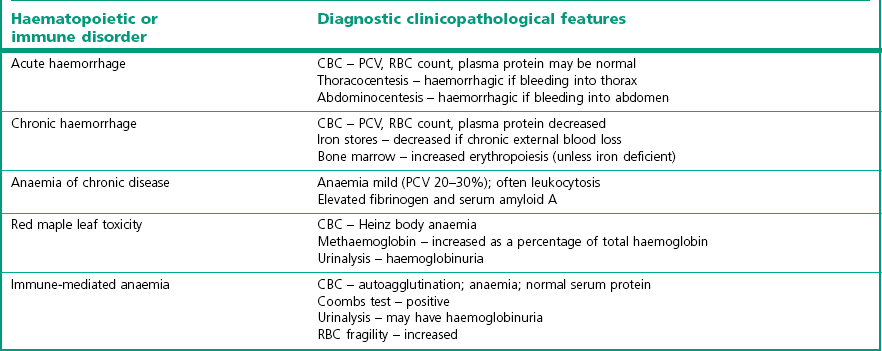
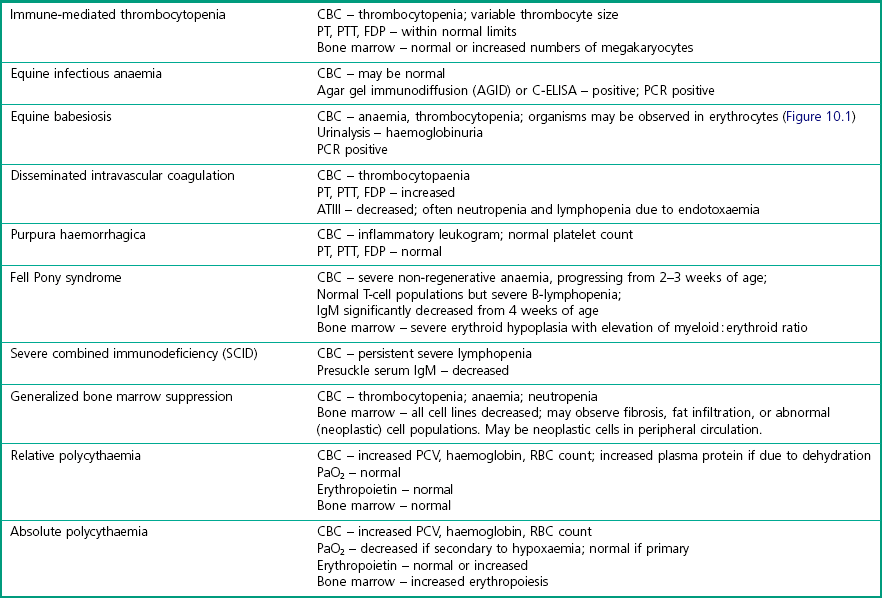
Chapter 10 10.1 Diagnostic approach to haematopoietic and immune system diseases 10.2 Diagnostic features of common haematopoietic and immune disorders of horses 10.3 Anaemia secondary to haemorrhage 10.4 Immune-mediated haemolytic anaemia 10.6 Heinz body haemolytic anaemia 10.7 Equine infectious anaemia 10.8 Equine piroplasmosis (babesiosis) 10.9 Anaemia due to rickettsial disease 10.11 Anaemia of chronic disease 10.12 Generalized bone marrow suppression or failure 10.13 Immune-mediated thrombocytopenia 10.14 Disseminated intravascular coagulation 10.16 Hereditary defects of haemostasis 10.20 Severe combined immunodeficiency (SCID) 10.22 Selective IgM deficiency 10.23 Transient hypogammaglobulinaemia 10.24 Failure of passive transfer 10.25 Neonatal isoerythrolysis 10.27 Lymphoma (lymphosarcoma) 10.29 Sporadic lymphangitis (‘monday morning leg’; ‘monday morning disease’) • Age – congenital abnormalities of the haematopoietic and immune system are most likely to become apparent in horses <1 year of age. Most congenital immunosuppressive disorders present before 3 months. • Breed – congenital abnormalities are also more likely in purebred horses, although they can occur in mixed-breed animals. • Specific questioning should be directed towards establishing: Presence of exercise intolerance? Change in demeanour, appetite or resting respiratory rate? Extent and rate of any weight loss. History of epistaxis, bleeding episodes or discolored urine? Appearance of limb swelling or unexplained masses? • In-contact horses – a history of similar clinical signs in other horses may suggest an infectious or toxic aetiology. A history of Streptococcus equi infection on the premises might suggest purpura haemorrhagica in a horse with vasculitis. • Season – red maple leaf toxicity is more common in the late summer and autumn. • Environment – fallen trees or poor pasture management might predispose to ingestion of toxic plants. Tick exposure is required for haemolytic anaemia due to piroplasmosis. • Routine herd health records – weight loss, ventral oedema, and unexplained recurrent fever in an untested horse might indicate equine infectious anaemia. Recent vaccination against Streptococcus equi might suggest a diagnosis of purpura haemorrhagica in a horse with vasculitis. • Previous illnesses – repeated incidences of infectious diseases may be suggestive of an immune dysfunction. Cryptosporidium spp., adenovirus infections, Candida spp. and Pneumocystis carinii infections have been diagnosed in horses with immunological disease. Physical examination: A complete physical examination is required to identify all clinical abnormalities. Specific diagnosis of a primary (rather than secondary) haematopoietic and/or immunological disease is then likely to require further diagnostic investigation. Mucous membranes: Change in mucous membrane colour is key to detection of disease and all available surfaces should be examined, including scleral colour. Pale mucous membranes accompanied by tachycardia, tachypnoea, and a systolic heart murmur are highly suggestive of severe anaemia. However, marked anaemia is required before changes in mucous membrane colour are readily detected. In contrast, congested mucous membranes with prolonged capillary refill time are consistent with polycythaemia. Petechial or ecchymotic haemorrhages occur with thrombocytopenia or vasculitis. Detection of petechiation in the presence of endotoxaemia may be the first indication of impending disseminated intravascular coagulation (DIC). Icteric sclera may be seen in haemolytic anaemia, but will occur also with prolonged inappetance. Haemorrhage: Often the source of blood loss is evident and a result of trauma. Intrathoracic haemorrhage (fractured rib/intercostal artery laceration) or intra-abdominal bleeding (splenic rupture, mesenteric tear) may require cytological evaluation of pleural or peritoneal fluid respectively or ultrasound examination. Prolonged bleeding from venipuncture sites, petechial or ecchymotic haemorrhages, or melaena occur with severe thrombocytopenia (usually <20 × 109/L). Haematoma formation following relatively minor trauma, or internal haemorrhage into the thorax or abdomen may be indicative of coagulation factor abnormalities or severe DIC. Palpation: Jugular veins should be palpated for patency, thickening, heat or painful foci. Jugular vein thrombosis associated with venipuncture is common in horses with endotoxemia or other conditions that cause blood to be hypercoaguable. Thrombosis may occur secondary to DIC, repeated or unskilled venipuncture or infection. Peripheral blood evaluation: A complete blood count (CBC) can give invaluable information about the erythron (red cell mass), the leukon (white cell mass) and platelets. Blood samples should be collected from any patent superficial vein when the horse has been calm for at least 1 hour. Catecholamine-induced contracture of the spleen in response to excitement causes a significant increase in red cell numbers. Catecholamine release also causes physiologic leukocytosis with increased neutrophil and lymphocyte counts. Similarly, samples should not be taken after ingestion of a large concentrate meal, as this will reduce circulating plasma volume. By contrast, sedatives such as acepromazine and xylazine cause splenic relaxation and relative anaemia such that blood samples should always be taken prior to sedation. Erythrocytes: The RBC count, haemoglobin, haematocrit and packed cell volume (PCV) are the most frequently measured parameters of erythrocyte quantitation. Haemoglobin concentrations should be approximately one-third of the PCV. This will be increased with intravascular haemolysis. Anaemia is a decrease in the circulating red blood cell (RBC) mass (decreased RBC count, haemoglobin and packed cell volume) as a result of decreased rate of erythrocyte production or increased rate of erythrocyte destruction or loss. Polycythaemia is an increase in the circulating RBC mass. Red blood cell indices can be used to characterize anaemia: • Mean corpuscular volume (MCV) – sometimes increased in horses with regenerative anaemia; decreased with iron deficiency anaemia. • Mean corpuscular haemoglobin (MCH) – increased with intravascular haemolysis; decreased with iron deficiency anaemia. • Mean corpuscular haemoglobin concentration (MCHC) – increased with intravascular haemolysis; decreased with iron deficiency anaemia. • Red cell distribution width (RDW) – may be increased in regenerative anaemia, reflecting anisocytosis. • Poikilocyte – any abnormally shaped erythrocyte. • Anisocytosis – varying sizes of erythrocytes are observed. • Polychromasia – varying colours of erythrocytes are observed, usually due to variable haemoglobin and RNA content. • Spherocyte – spherical erythrocytes occasionally observed in horses with immune-mediated anaemia. • Howell–Jolly bodies – basophilic nuclear remnants occasionally observed in the cytoplasm of erythrocytes of normal horses. • Heinz bodies – oxidized precipitated haemoglobin observed as round structures extending from the edge of the RBC membrane. Indicative of oxidative damage to the RBC with subsequent intravascular or extravascular haemolysis. A reference range for haematology values in the adult horse is given in Table 10.1. Table 10.1 Reference range for haematology values in the adult horse Leukocytes: Both total WBC count and differential WBC counts should be assessed. • Neutropenia – decreased neutrophil count – most often a result of a shift of neutrophils from the circulating to the marginating pools due to excessive tissue demand. This may occur with endotoxaemia, septicaemia, and a wide variety of acute bacterial, rickettsial or viral disorders. In endotoxaemia the neutrophils develop basophilic cytoplasm, with granulation, vacuolation and presence of dark-staining Dohle inclusion bodies (‘toxic neutrophils’). Chronic neutropenia may occur with severe infectious or inflammatory diseases or bone marrow suppression. • Band neutrophils – are immature neutrophils released prematurely into the circulation as a response to high tissue demand (‘left shift’). A degenerative left shift occurs when the number of immature cells exceeds the number of mature neutrophils because of an inability of the bone marrow to keep pace with demand. It is considered a poor prognostic indicator. • Neutrophilia – increased neutrophil count – may occur in response to stress, excitement, exercise, glucocorticoid or adrenaline (epinephrine) administration, or almost any infectious or inflammatory condition. May be accompanied by a regenerative left shift. Often follows neutropenia caused by endotoxaemia or infection. • Eosinopenia – decreased eosinophil count – may be normal or may occur in response to stress or glucocorticoid administration. • Eosinophilia – increased eosinophil count – occurs in response to some parasitic infections or hypersensitivity reactions e.g. allergic respiratory disease; allergic dermatitis. It may also occur as a primary myeloproliferative disease, or in response to systemic neoplasia. • Basophilia – increased basophil count – may be observed in response to allergic, inflammatory, or neoplastic diseases or in association with lipaemia. (Basopenia is not clinically significant.) • Monocytosis – increased monocyte count – occasionally observed in association with chronic inflammation. (Monocytopenia is not clinically significant.) • Lymphopenia – decreased lymphocyte count – occurs in response to stress, glucocorticoid administration, many acute viral infections, endotoxaemia and in foals with severe combined immunodeficiency (SCIDs). • Lymphocytosis – increased lymphocyte count – associated with excitement, exercise, adrenaline (epinephrine) administration, lymphocytic leukaemia, and chronic antigenic stimulation. Platelets: Total counts in the horse are usually less than in other species. Platelets may clump if they have become activated in vivo or in vitro. Clumped platelets may be observed at the feathered edge of a stained blood smear, particularly with EDTA samples. If platelets have clumped, automated counts are not accurate. A repeat sample should be collected in sodium citrate and counted for more accurate data. Below 100 × 109/L thrombocytes is of clinical significance, but frank haemorrhage is unlikely to occur unless platelet numbers fall below 20 × 109/L. Splenic contraction will raise numbers. • Autoagglutination – agglutination of erythrocytes that is not eliminated by diluting blood in sterile saline is suggestive of immune-mediated haemolytic disease (dilute 1 drop EDTA blood: 4 drops saline). • Coombs test – direct Coombs test detects antibody and/or complement on the surface of erythrocytes; indirect Coombs test detects the presence of circulating anti-erythrocyte antibodies. Either or both may be positive in immune-mediated haemolytic disease. • Osmotic fragility test – assay assesses stability of erythrocytes in saline solutions of decreasing tonicity. Increased fragility is associated with immune-mediated haemolytic disease. • Flow cytometry – flow cytometric detection of platelets with surface-bound IgG can be used to identify cases of immune-mediated thrombocytopenia (IMT). Diagnosis of IMT otherwise depends on elimination of other causes of thrombocytopenia. • Cross-matching – major cross-match checks for compatibility between blood donor erythrocytes and any alloantibody present in patient serum. The minor cross-match checks for compatibility between donor serum and patient erythrocytes. Both tests are important prior to whole blood transfusion. The minor cross-match is most important prior to plasma transfusion. • Iron status – may be important in characterization of some anaemias. Serum ferritin concentration is indicative of stored hepatic and splenic iron. Prussian blue stain of bone marrow aspirates also allows assessment of iron stores. Serum iron concentration reflects total quantity of transport iron in the plasma (iron bound to transferrin). Total iron binding capacity (TIBC) is the amount of iron that plasma transferrin could bind if fully saturated. Percentage transferrin saturation is calculated from TIBC and serum iron concentration and should normally be approximately 30%. Bone marrow evaluation: Bone marrow aspirates and core biopsies may be obtained from the sternebrae on the ventral midline between the forelimbs, from the tuber coxae with the needle directed toward the opposite coxofemoral joint, or from the proximal ribs. The area is clipped and surgically scrubbed. A small amount of local anaesthetic is deposited in the subcutaneous tissues and periosteum. A stab incision is made with a small surgical blade. A 16-gauge or larger bone marrow needle is inserted and forced into the marrow cavity with constant pressure and rotational movement. The stylet is removed and a sterile syringe containing a small amount of EDTA or sodium citrate as anticoagulant is used to aspirate material. Smears should be made immediately and stained for cytological analysis. Core biopsies are similarly obtained using a 10-gauge Jamshidi needle and fixed for routine histopathological analysis. Evaluation of haemostatic function: Evaluation of haemostatic function should include a total platelet count, tests to evaluate the function of extrinsic and intrinsic coagulation cascades, and assays to estimate fibrinolytic activity. Samples should be collected in a citrate tube and values compared to a healthy control horse. Activated partial thromboplastin time (APTT) measures the function of the intrinsic and common coagulation pathways. Prolonged PT with a normal APTT indicates a deficiency of factor VII in the extrinsic pathway (e.g. in hepatic failure). Prolongation of both PT and APTT is more likely to occur with poisoning (e.g. vitamin K antagonists) or DIC. Mucous membrane changes in a horse with DIC are shown in Figure 10.3. Platelet count <10 000/µL is associated with haemorrhage (Figure 10.2). Platelet function may be evaluated with template bleeding time assays or platelet aggregation tests. • Urinalysis – haemoglobinuria occurs with intravascular haemolysis. Haematuria with erythrocytes in urine sediment occurs with urinary tract haemorrhage and/or inflammation. • Thoracocentesis – may be useful for identification of intrathoracic haemorrhage. Abnormal lymphocytes observed on cytology is compatible with a diagnosis of thoracic lymphoma. • Abdominocentesis – may be useful for identification of intra-abdominal haemorrhage. • Testing of faecal occult blood may help to indicate if gastrointestinal or respiratory tract haemorrhage has occurred but tests are considered to be unreliable. Immunoglobulin assays.: Screening tests to assess for gross alterations in serum IgG concentration include zinc sulphate turbidity, glutaraldehyde coagulation, latex agglutination, or enzyme linked immunosorbent assay (ELISA). More accurate quantification is available with radial immunodiffusion (RID) techniques. RID test kits are available to accurately quantitate serum IgG, IgM, IgG(T) and IgA. Production of antibody in response to vaccination with a specific antigen may be used to assess responsiveness of the humoral immune system. Lymphocyte typing.: Peripheral lymphocyte counts are extremely low in Arabian foals with severe combined immunodeficiency (SCID). Dramatically increased counts with abnormal cell morphology may be observed in horses with lymphocytic neoplasias. Monoclonal antibodies are available for identification of lymphocyte subsets (CD4, CD8, pan T cell, B cell) in peripheral blood by flow cytometry. Staining of tumours with antibodies against CD3 and CD79 has been used to classify T-cell and B-cell tumours respectively. Blood typing.: Several laboratories perform erythrocyte alloantigen typing and alloantibody detection for prevention or diagnosis of neonatal isoerythrolysis (NIE). These laboratories will also perform lymphocyte typing for progeny verification. NIE is more likely to occur in the progeny of horses with blood groups Aa or Qa. Cellular immune function.: Intradermal skin testing with phytohaemagglutinin (PHA) may be used to assess delayed type hypersensitivity. A crude estimate of T cell function may be obtained with lymphocyte blastogenesis assays using PHA, concanavalin A or pokeweed mitogen. More specific tests may be available in some research laboratories. Haemorrhage: aetiology and pathogenesis: Blood loss or haemorrhage may be external or internal, acute or chronic. • External blood loss is often a result of trauma and easily recognized and diagnosed with a visual examination. An exception to this is haemorrhage into the gastrointestinal tract. Significant haemorrhage into the gastrointestinal tract may occur with gastric ulceration or intestinal neoplasia without evident melaena or haematochezia. • Internal blood loss, into the thorax or abdomen, allows the body to re-use (autotransfuse) up to two-thirds of the erythrocytes lost. Common causes include severe exercise induced pulmonary haemorrhage (EIPH), post partum rupture of the uterine artery and splenic rupture subsequent to trauma. • Acute blood loss – often associated with normal RBC parameters because all blood components have been lost in equal volumes. Redistribution of fluid from interstitial tissue into the intravascular space results in a detectable drop in total plasma protein after 6 hours. Anaemia only becomes apparent after 12–24 hours, with the lowest values in PCV occurring at 36–48 hours post haemorrhage. Bone marrow exhibits increased erythropoiesis by 3 days post haemorrhage; maximal by 10 days. Horses do not release reticulocytes into the circulation during a regenerative response. PCV should increase by 0.32–0.42% per day during maximum regeneration. Complete restoration of circulating red cell count may require 1 to 3 months. Weekly assessment of PCV and TP aids monitoring of regeneration process. Serial bone marrow aspirates must be examined if regenerative responses are questionable. Clinical signs: Signs depend on the source of haemorrhage, plus the rate and total volume of blood loss. Mares with post partum uterine artery rupture for example show signs of colic followed by progressive tachypnoea, tachycardia and pallor. Signs of hypovolaemic shock develop when horses lose more than 30% of circulating blood volume (i.e. 24 mL/kg or 9 L of blood for a 400 kg horse). Signs are more subtle in cases of chronic blood loss due to physiological adaptation, such that vital parameters may only be elevated when the horse is exercised or stressed. Packed cell volume may decrease as far as 8–10 L/L in chronic cases before clinical signs become readily apparent at rest. A summary of clinical signs is shown in Table 10.3. Table 10.3 Signs of acute and chronic blood loss
Haematopoietic and immune systems
10.1 Diagnostic approach to haematopoietic and immune system diseases
Haematology value
Reference range
Red cell count (RBC), × 1012/L
6.5–11.6
Packed cell volume (PCV), L/L
0.30–0.48 (30–48%)
Haemoglobin, g/dL
11.3–17.9
Mean corpuscular volume (MCV), fl
39–49
Mean cell haemoglobin (MCH), pg
12.3–17.0
Mean corpuscular haemoglobin concentration (MCHC), g/L
31.0–38.6
Red cell distribution width (RDW), %
<20.0
Leukocytes (WBC), × 109/L
5.3–11.0
Neutrophils, × 109/L
2.1–6.0
Band neutrophils, × 109/L
0.0–0.2
Lymphocytes, × 109/L
1.7–5.4
Neutrophil : lymphocyte ratio
1.5 : 1.0
Monocytes, × 109/L
0.0–0.7
Eosinophils, × 109/L
0.0–0.8
Basophils, × 109/L
0.0–0.1
Platelets, × 109/L
100–350
10.3 Anaemia secondary to haemorrhage
Acute blood loss
Chronic blood loss
Hypovolaemic shock
May be anorexia and weight loss
Tachycardia
Decreased exercise tolerance
Tachypnoea
Pale mucous membranes
Pale mucous membranes
Weakness, lethargy
Poor venous distension
Tachycardia when stressed
Thready pulse
Tachypnoea when stressed
Pansystolic heart murmur
Vitals may be normal at rest
Weakness, lethargy
Pansystolic heart murmur
Renal hypoxia: oliguria
Acute renal failure
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree