33 Haematological and haemostatic emergencies
Canine Immune-Mediated Haemolytic Anaemia (IMHA)
Theory refresher
IMHA is generally classified into primary (idiopathic) IMHA, in which no inciting cause is identified, and secondary IMHA, which is associated with an underlying condition (Box 33.1). Primary IMHA is typically diagnosed in young or middle-aged adult dogs but can occur in older dogs too. IMHA is especially common in certain breeds (e.g. English springer spaniel, American Cocker Spaniel). These dogs were previously classified as having primary IMHA although this classification may no longer be appropriate given their genetic predisposition. In dogs with secondary IMHA, the underlying condition may or may not persist at the time of presentation, and may or may not be amenable to specific therapy. In the absence of useful historical information (e.g. with respect to drug administration or vaccination), thorough physical examination, thoracic and abdominal diagnostic imaging, urine microbiology and additional investigations as appropriate are required to rule out an underlying trigger for IMHA.
BOX 33.1 Underlying conditions potentially associated with immune-mediated haemolytic anaemia
FeLV, feline leukaemia virus; FIV, feline immunodeficiency virus.
Treatment
Medical therapy to decrease erythrocyte phagocytosis, complement activation and anti-erythrocytic antibody production is essential for primary IMHA and glucocorticoids are the mainstay of acute therapy. Other immunosuppressive agents may be used in addition to, but not instead of, glucocorticoids (Box 33.2). Immunosuppressive therapy is typically also required for secondary IMHA although success is significantly dependent on whether the underlying condition persists and, if so, whether or not it is amenable to treatment. Clearly with neoplastic conditions in particular, it may not be possible or appropriate to treat the patient.
Case example 1
Emergency database
An intravenous catheter was placed in a cephalic vein and blood obtained via the catheter for an emergency database to be performed. This revealed severe anaemia (manual packed cell volume (PCV) 14%, reference range 37–55%) with raised plasma total solids (78 g/l, reference range 49–71 g/l); however, the serum was icteric in appearance. These findings were consistent with the suspicion of haemolytic anaemia. An in-saline agglutination test was performed (Box 33.3) and found to be strongly positive (Figure 33.1). Peripheral blood smear examination revealed a strongly regenerative anaemia with marked polychromasia and anisocytosis and an increase in nucleated red blood cells; marked spherocytosis was also identified. Platelet numbers were adequate, and a moderate subjective left-shifted neutrophilia and a monocytosis were suspected (see Ch. 3).
BOX 33.3 In-saline agglutination test for autoagglutination
One or more drops of fresh blood (or blood from an EDTA tube) are placed on a glass slide and an equal volume of normal saline added. The slide is gently rotated and then examined for evidence of macroscopic agglutination after approximately 1 minute. The slide is then examined microscopically as well (Figure 33.1). Saline is added to differentiate true agglutination from rouleaux formation. Rouleaux are chains of red blood cells stacked like coins, and are especially common in cats and animals with inflammation. They should be dissipated by the addition of saline.
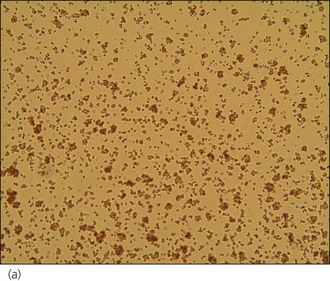
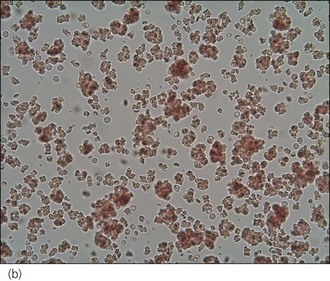
Figure 33.1 Microscopic in-saline agglutination (a) ×100 and (b) ×200 magnification.
(Photographs courtesy of Kate English)
Clinical Tip
Clinical Tip
Case management
The dog was started on immunosuppressive therapy as follows:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


