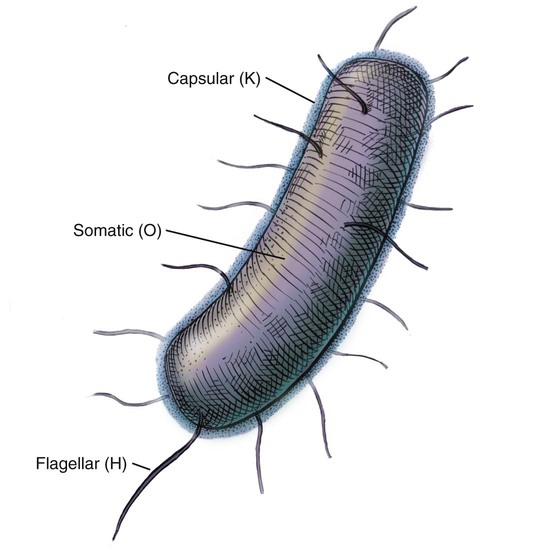
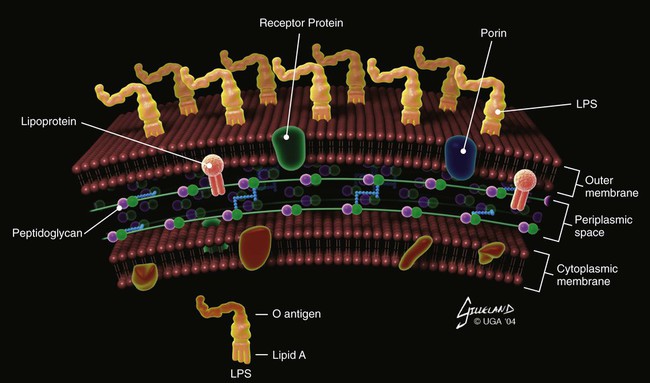
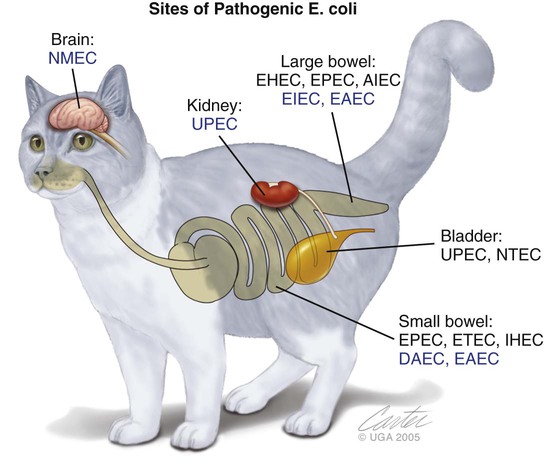
The cell wall of each gram-negative bacterial species has evolved a relatively complex outer membrane in addition to its inner cytoplasmic membrane and intermediate peptidoglycan layer. This outer membrane distinguishes the group from gram-positive bacteria and spirochetes. The outer surface of this membrane consists predominantly of a lipopolysaccharide (LPS, or endotoxin), which has a lipid portion embedded in the membrane (lipid A—the active component of endotoxin) and a polysaccharide portion extending from the bacterial surface (the O antigen; Fig. 35-1). In addition to conferring a gram-negative staining characteristic, these structures are important virulence factors and partially determine antibacterial susceptibility. Gram-negative bacteria fall into numerous heterogeneous phylogenetic groups, and many can be pathogenic in dogs and cats. Some of these bacteria are discussed in other chapters: Pasteurella spp. (see Chapters 50, 51, and 99), Bordetella bronchiseptica (see Chapters 6 and 14), and Salmonella spp. (see Chapter 37). This chapter focuses on the general characteristics of Escherichia coli, Proteus spp., Klebsiella spp., and Pseudomonas spp. infections. Information is also provided for Ralstonia pickettii, Citrobacter spp., Acinetobacter baumannii, Providencia spp., Serratia spp., and Enterobacter cloacae infections. Pseudomonas spp. are ubiquitous, non-Enterobacteriaceae, gram-negative bacteria that are found in soil, water, decaying vegetation, and animals. The most important and ubiquitous pathogenic pseudomonad is Pseudomonas aeruginosa. In Asia, Eastern Europe, and Africa, Pseudomonas mallei is the cause of equine glanders; dogs and cats can become infected from the horse reservoir (see Chapter 44). Burkholderia (formerly Pseudomonas) pseudomallei is a soil organism found in Asia, central Africa, and Australia. In dogs and cats, it can cause syndromes ranging from a benign pulmonary infection to a lethal systemic disease (see Chapter 44). An internationally recognized serologic classification scheme has been developed for the Enterobacteriaceae based on antigenic differences in highly variable bacterial surface molecules. The serogroup is determined by the O antigens—sugars on the most external part of LPS (Fig. 35-2). The serotype is determined by flagellar H antigens. Additionally, K antigens can be identified in strains with a capsule. The antigenic formula of the strain indicates the presence of specific antigens (e.g., O55:K5:H21). However, for most strains, only O and H antigens are determined. At least 170 different serogroups have been identified for E. coli, and some correlation has been found between serogroup and virulence (e.g., O157:H7 and hemolytic uremic syndrome (HUS) in humans). The bacteria are commonly isolated from normal epithelia and spontaneously arising clinical infections of several different organ systems of dogs and cats (Web Table 35-1). They can cause sepsis, nosocomial infections, and opportunistic infections in immunocompromised animals and accounted for approximately 30% of positive blood cultures in one study.51 Isolates can be endogenous or exogenous to the individual. These types of infections may be increasing in frequency because of the overuse of new antibacterials, invasive procedures, and immunosuppressive therapies. WEB TABLE 35-1 Examples of Isolation and Infection Sites of Enterobacteriaceae and Pseudomonas Bacteria aTissue from which E. coli, Klebsiella, Proteus, or Pseudomonas or all of these bacteria can be cultured. Some strains of E. coli are true pathogens; however, most E. coli, Klebsiella, Proteus, and Pseudomonas organisms are opportunistic pathogens. The pathogenicity of gram-negative bacteria depends on the presence of various virulence factors (e.g., adhesins, toxins, iron-acquisition systems, capsules) expressed by the organism.25a It is the set of virulence genes carried and expressed by the organism that confers pathogenicity rather than the genus or species designation. Examples of virulence factors follow. Production of various toxins is important in the pathogenesis of gram-negative infections. Secreted α-hemolysin damages host cell membranes. Endotoxin (LPS) stimulates various cytokine cascades, as well as the complement cascade. Excessive activation of these systems leads to sepsis and septic shock (see Chapter 36). Pseudomonas spp. variably produce collagenase, lecithinase, lipase, proteases, hemolysins, fibrinolysin, leukocidin (also called cytotoxin), and enterotoxin. Many strains also secrete exotoxin A, a cytotoxin that causes tissue necrosis, allowing the organism to colonize deep tissues in burn and trauma cases. As with the Enterobacteriaceae, Pseudomonas LPS can act as an endotoxin. The documentation of pathogenic E. coli infections is confusing because many of these organisms exist as normal enteric constituents and can be found in the microflora of clinically healthy animals. Analysis for specific toxin genes or molecular typing can be done to differentiate among the different isolates. Toxin analysis is important in addition to genetic studies because many of the genes identified may not be expressed by the organisms isolated. Pathogenic E. coli have been classified on the basis of their virulence properties into pathovars. Diarrheagenic pathovars include enteropathogenic E. coli (EPEC), enterohemorrhagic E. coli (EHEC), enterotoxigenic E. coli (ETEC), enteroinvasive E. coli (EIEC; including Shigella), enteroaggregative E. coli (EAEC) and diffusely adherent E. coli (DAEC), necrotoxigenic E. coli (NTEC), adherent invasive E. coli (AIEC), and cell-detaching E. coli (CDEC). Extraintestinal pathogenic E. coli (ExPEC) pathovars include uropathogenic E. coli (UPEC) and neonatal meningitis E. coli (NMEC) (Table 35-1, Web Table 35-2). Not all pathovars have been documented in dogs and cats (Fig. 35-3). Pathovars are not clear-cut distinctions; some enteropathogens isolated from dogs have predominantly “uropathogenic” traits, but they cause diarrhea. The pathovars are discussed in the following sections. TABLE 35-1 Pathogenic and Clinical Characteristics of Pathogenic Escherichia coli Strains WEB TABLE 35-2 Pathogenic and Clinical Characteristics of Pathogenic Escherichia coli Strains EPEC adhere to mucosal cells of the small intestine and colon causing loss of microvilli (attaching and effacing lesions) and the formation of filamentous actin pedestals or cuplike structures under the organisms. The locus of enterocyte effacement (LEE) encodes a type III secretion system (T3SS) that facilitates translocation of effectors, including the intimin receptor (Tir). EPEC adherence to cells via the bundle-forming pilus is mediated by binding of Tir and the bacterial outer-membrane protein intimin, essentially forcing the host cell to form the attachments to the bacteria using its own actin. Other effectors translocated by T3SS undermine normal host cell functions in many ways, such as by rearranging cytoskeleton, modulating the immune response, and contributing to diarrhea. Mitochondrial-associated protein (Map), for example, disrupts mitochondrial function. The secreted protein EspF triggers mitochondrial death and may also inhibit phagocytosis and interrupt cellular tight junction. Several other effectors (EspB, EspI) alter protein trafficking and prevent mitosis or induce apoptosis (cycle inhibiting factor, Cif). EPEC also occasionally invade mucosal cells. Clinically, EPEC cause watery diarrhea as a result of host cell invasion and alteration of signal transduction systems rather than as a result of production of toxin.10,24,24 Fifteen EPEC strains were isolated from 300 cats in Brazil; only one cat had diarrhea. Twelve different EPEC serotypes were identified, including two that are known human pathogens (O111:H25 and O125:H6).98 Twenty-three EPEC isolates were cultured from 182 fecal specimens from 146 dogs with diarrhea and from 36 dogs without diarrhea in Brazil. There was no statistical difference between percentage of EPEC-positive samples in dogs with (13.7%) or without (8.3%) diarrhea. Many serotypes were identified, including several typical and atypical human EPEC serotypes.100 EHEC bind tightly to epithelial cells and produce the same type of attachment-effacement lesions as EPEC, preferentially localizing in the distal ileum and colon. The initial binding may occur through a type IV pilus called the hemorrhagic coli pilus that functions in adherence and biofilm formation; binding may also occur via the E. coli common pilus and flagella.24 Intimate attachment occurs via the same LEE as EPEC, although EHEC inject nearly twice as many effectors as EPEC. EHEC also form pedestals beneath the organisms, although the mechanism for pedestal formation is slightly different than in EPEC, because the Tir is not phosphorylated by the host cell. EHEC produce two major antigenic forms of toxin called Shiga toxins (Stx 1 and Stx 2 [also known as verocytotoxins (VT)-I and VT-II]) or Shiga-like toxins (SLT). EHEC lack the ability to secrete Stx. Within the bacteria, phages harbor Stx genes. Stx is released on lysis of the bacterium, which is mediated via phages in response to bacterial DNA damage. This so-called SOS response is regulated by the repressor enzyme, LexA, and recombinase, RecA. Use of antimicrobials such as quinolones should be discouraged, because they may lead to even more toxin release, allowing infection and replication within susceptible cells.125 Stx is an AB5 toxin, composed of an enzymatically active subunit A covalently bound to five B subunits. The B subunit binds to globotriaosylceramides (Gb3s) found on intestinal mucosa and kidney epithelial cells and induces the cell to internalized the toxin. Subunit A then prevents protein synthesis by interfering with 28S ribosomal RNA (rRNA) and causes necrosis and death of the cell. Stx can also be found in Gb3 negative intestinal cells and is thought to enter the cells via micropinocytosis. Stx in these cells is believed to reduce chemokine expression, thus reducing the inflammatory response. E. coli O157:H7 (often called verotoxigenic E. coli, VTEC) is an EHEC serotype responsible for foodborne and waterborne infection in people, particularly in North America, parts of Europe, and Japan. In people, EHEC infections have been associated with diarrhea, hemorrhagic colitis, and HUS. In almost all humans the toxin-producing organism has originated from the gastrointestinal (GI) tract; however, one report includes a urinary source of infection.139 The organism is harbored by cattle but does not cause them to develop any clinical illness, because cattle do not possess the Stx receptors. Large numbers of cattle tested at slaughterhouses harbor this bacterium; however, not all strains carry the virulence factors incriminated in severe disease. Undercooked beef and water contaminated by feces from infected animals or people are the most common sources for spread. Dogs living on farms and feed lots have also been documented as harboring E. coli O157:H7.56,73,74,125,144 The dogs were not clinically ill, and the organism was isolated from their feces. Infected cattle were considered the source of infection. Infection was also documented in a pet dog that was fed food scraps116 and in dogs frequenting beaches.38 In all these reports the dogs were asymptomatic for infection, and the public health risk was of most concern. EHEC have been isolated from stools of normal dogs and cats and those with diarrhea. A case-control study suggested that Stx 2 (VT-II)-positive isolates may be causally associated with diarrhea in dogs, whereas Stx 1 (VT-I)-positive isolates are carried but do not appear to cause diarrhea.10 In kenneled greyhounds, enterotoxin genes were identified in E. coli from dogs with and without diarrhea; however, only the presence of Shiga toxin Stx l correlated with diarrhea.134 The presence of toxigenic E. coli in clinically healthy and diseased dogs indicated a potential zoonotic risk. An epidemiological study of dogs and cats in Japan produced only one O157:H7 isolate from 614 samples, suggesting that these species are not a common reservoir.73 Cats in Ontario, Canada, were examined to determine the prevalence of VTEC in feces related to the presence or absence of clinical disease.132 Organisms were screened for VT genes by the polymerase chain reaction (PCR); the overall prevalence of enteric VTEC in 179 cats was 12.3%. However, no statistical association was found between the presence of the bacteria and clinical illness. Cats were positive for generic VT but not for VT-I or VT-II genes, suggesting that the VT may have been a new, nonpathogenic variety. Some of the isolated serotypes were similar to those found in people and cattle, suggesting that cats might be capable reservoirs for human infection.132 ETEC is a common cause of “travelers’ diarrhea” and has a significant impact on the swine industry, because postweaning pigs are highly susceptible. The bacteria adhere to small intestinal mucosal cells and produce diarrhea by elaborating toxins. Initial binding to intestinal epithelium occurs via colonization factors, which can be fimbrial, nonfimbrial, helical or fibrillar, and via flagella that are transiently bound with the secreted adhesion EtpA. More intimate attachment is via outer-membrane proteins Tia and TibA.24 Little inflammation and usually no histologic changes develop in mucosal tissues to which the bacteria are attached. Heat-labile toxins (LTs) and heat-stable toxins (STs) are well characterized. LTs (LT-I, LT-II) increase adenyl cyclase activity in epithelial cells of the small intestine. This ultimately results in inhibition of sodium and chloride absorption in villous epithelial cells and in active secretion of chloride by crypt cells, causing extensive loss of water and chloride. Data conflict about whether LT-producing E. coli have been isolated from dogs.10,34,55,103,146 STs (STa, STb) are a family of small toxins, some forms of which deregulate guanylyl cyclase, with resultant fluid secretion similar to that induced by LTs. Other forms stimulate cyclic nucleotide-independent secretion. ST-secreting E. coli have been isolated from dogs and cats with enteritis, supporting a causal association.10,55 NTEC produce cytotoxic necrotizing factors (CNFs) and cytolethal distending factor. CNFs are protein toxins that were originally called “vir toxin.” CNF1 and CNF2 have been associated with diarrhea, bacteremia, and urinary tract infections (UTIs) in humans. E. coli producing CNF1 have been isolated from stools of normal dogs and cats, as well as dogs (but not cats) with enteritis.10,137 Serogroups of NTEC-1 strains have been the same as those that infect people.30 NTEC may have been responsible for rhabdomyolysis in a cat with chronic renal failure and an episode of acute enteritis and was isolated from the lungs of a dog with fatal peracute hemorrhagic pneumonia.16,89 An association has been made between histiocytic ulcerative (granulomatous) colitis in boxer dogs and intramucosal colonization by E. coli that phylogenetically resembles Crohn’s disease–associated E. coli LF-82 and has a virulence gene composition that resembles extraintestinal pathogenic E. coli and Crohn’s disease–associated E. coli LF-82. All strains were positive for the adhesin-encoding gene associated with epithelial cell invasion in UPEC (fimH) and were negative for a variety of diarrheagenic and ExPEC virulence factors.130 AIEC has been implicated in approximately 37% of human Crohn’s disease. It adheres to carcinoembryonic antigen-related cell adhesion molecule 6 in the ileum, which is overexpressed in patients with Crohn’s disease. Adherence stimulates tumor necrosis factor (TNF)-α and interferon-γ, which may promote inflammation and adherence. AIEC translocate into the lamina propria, where they live and replicate within macrophages, stimulating production of large amounts of TNF-α. Granuloma formation is thought to occur due to aggregation and fusion of infected macrophages with subsequent recruitment of lymphocytes (see also Granulomatous Colitis, Chapter 88).120 EIEC and Shigella share the same pathogenicity. Shigellosis is a disease causing bloody diarrhea and bacillary dysentery in humans. EIEC differ from other pathovars because they are obligate intracellular bacteria that have neither flagella nor adherence factors to facilitate cell entry. EIEC and Shigella virulence is encoded on a large plasmid. Via transcytosis, the organism passes through colonic microfold cells (M cells) to the submucosa. They may also gain access to the submucosa subsequent to inflammation and interruption of cell tight junctions. EIEC/Shigella then enter and replicate within macrophages, are released from dead macrophages, and then invade adjacent colonocytes. Effectors secreted into the cell by a T3SS are responsible for subversion of the host cell machinery to facilitate replication, bacterial spread cell to cell, and evasion of the immune system.24 Some strains of E. coli can cause extraintestinal infections, including UTIs, pneumonia, sepsis, and meningitis; these strains are designated ExPEC, in keeping with the terminology used for strains causing enteritis. This classification has some overlap, and some E. coli isolated from dogs with diarrhea have characteristics of uropathogenic and necrotoxigenic strains.137 E. coli are a frequent cause of extraintestinal and extra–urinary tract infections.48 Such isolations include a report of E. coli endocarditis in a cat28 and E. coli isolated from a diabetic dog with emphysematous cystitis, emphysematous peritonitis, and infective endocarditis of the tricuspid valve.107 E. coli was also implicated as a cause of necrotizing fasciitis associated with a skin laceration in a dog.161 ExPEC has also been implicated in an outbreak of acute fatal necrotizing pneumonia in shelter cats. The isolates were hemolytic, genetically related as determined by PCR and carried genes for CNF-1 and fimbriae and adhesions that are characteristic of ExPEC.138 Infection has also been documented in three isolated cases in kittens, each with a distinct serotype (O4H5, O6H7, O6H5) and carrying genes consistent with ExPEC.61 An outbreak of fatal hemorrhagic pneumonia and bacteremia in kenneled dogs was attributed to a pathogenic E. coli that was positive for virulence factors α-hemolysin and CNF-1 and for the adhesin factor class-III papG allele.57 These organisms have the phenotypic and genotypic characteristics of extraintestinal virulent bacteria that are a risk for people and other animals. Cultures of fecal samples collected from a clinically healthy cohort population of kittens revealed that 16 of 19 tested kittens were shedding a total of 10 different serotypes of hemolytic E. coli, each of which had a genetic profile consistent with that typical of ExPEC.61 E. coli is the most common isolate from dogs with first-time and recurrent UTIs and the second most common isolate from dogs with uroliths.83,102 Some uropathogenic strains of E. coli, designated UPEC, express virulence factors that facilitate the colonization of the urinary system. The major determinant of their pathogenicity is the ability to adhere to the uroepithelium via adhesins such as type 1 pili (mediated by fimbrial adhesion H at its tip) or P fimbriae, the gene of which (papG) has been identified in canine isolates.69,71 After attachment, pili and endotoxin incite neutrophil migration and induce the release of cytokines, leading to an inflammatory response and characteristic signs of urgency, discomfort, and hematuria (see Chapter 90). Exotoxins, especially hemolysins, may also be important in the development of clinical signs of lower UTIs. Iron acquisition is also an important virulence factor, as are bacterial capsules and antiphagocytic systems. Numerous virulence factors have been identified in canine and feline UPEC strains, including CNF1 and genes encoding adhesins, hemolysin, and aerobactin.37,163 The ability of some UPEC to actually invade uroepithelial cells has been reported in strains isolated from people. UPEC can replicate and form protective biofilm-like complexes within the cells. It is possible that this characteristic internalization of the organism may explain some relapsing UTIs in dogs and cats. UPEC are carried in the colons of many dogs; comparing phylogenetic background, O antigens, and extended virulence genotype, 54% of urinary isolates were also found in the gut. This is consistent with the idea that UPEC have a special type of pathogenicity, and UTIs are not the result of exposure to high numbers of “normal” E. coli.68 Hemolytic E. coli have also been found in UTIs in cats.143 Untreated UPEC cystitis can progress to pyelonephritis if the organism is motile and motility and the degree of type 1 pili expression have been shown to be inversely related. Within the kidney, pyelonephritis isolates often express P fimbriae and fewer motility organelles.78,79,81,129 Studies based on the identification of genes encoding adhesins, hemolysins, CNF1, and aerobactin suggest that canine uropathic strains may be similar if not identical to some human uropathic strains.71 Similarly, P. mirabilis strains isolated from the urine of dogs have been characterized and found to be similar to human uropathogenic P. mirabilis strains. The significance of these findings for people and dogs is not known.44 ExPEC can also infect almost any anatomic site. Pneumonia, sepsis, and meningitis are some of the infections that have been observed. Multidrug-resistant strains have been isolated from animals with recurrent infections that have been treated with several types of drugs.142 Klebsiella organisms, which are found in the nasopharyngeal and intestinal flora, can be associated with clinically significant infections of the GI and genitourinary tracts and systemic bacteremia. Some strains of Klebsiella produce an enterotoxin similar to the ST of enterotoxigenic E. coli. Klebsiella pneumoniae–induced enteritis, pneumonia, and sepsis were reported in a Bordeaux mastiff breeding kennel. Seven of 15 adult dogs were affected and 4 died.118 Klebsiella pneumonia and septicemia with subsequent multiple organ dysfunction syndrome has also been reported in a dog.20 Klebsiella organisms have been implicated in nosocomial infections, including intravenous catheter infections, in veterinary hospitals (see Chapter 93).49 P. aeruginosa is an environmentally stable organism that causes infection in animals whose immune systems or host defenses are impaired. Although it can be isolated from the upper respiratory, genital, and GI tracts and from intertriginous areas of the skin, numbers of organisms are usually controlled by competition from normal bacterial flora. Inflammation or injury to these mucosal areas or chronic antibacterial therapy can lead to a proliferation of P. aeruginosa. P. aeruginosa preferentially binds and enters the injured cells via the basolateral surface. The bacterium has been shown to facilitate its entry into cells by constitutively turning apical membranes into basolateral membranes by recruiting phosphatidylinositol-3-kinase and actin to the basolateral surface.75 In addition, open wounds or surgical incisions can become contaminated and subsequently infected with antimicrobial-resistant strains from the hospital environment. Defective flexible endoscopes that became contaminated were incriminated as a source of an outbreak in people.134 Pseudomonas bacteria also have the ability to invade a healthy cornea in its warm, moist state. Through small scratches, the organism can enter the cell cytoplasm, where it rapidly replicates. However, some strains produce an exoenzyme toxin that kills epithelial cells and macrophages. In dogs with otitis externa, P. aeruginosa and Proteus organisms could be cultured from the ears but were not found in the ears of clinically healthy dogs.163 P. aeruginosa has been implicated as a cause of deep pyoderma in dogs, sometimes manifesting as acute onset dorsal truncal pain.62 Osteomyelitis has also been reported.107a Pseudomonas bacteria are also isolated from mixed infections in dogs.136 P. aeruginosa, which has few nutritional requirements, thrives in moist environments outside the host and creates microcolonies that allow it to grow on moist surfaces such as those in bath tubs and plumbing and drainage systems. Animals that are immunosuppressed or have implanted catheters or open wounds are particularly susceptible to contamination from these moist environments or surfaces. In hospital environments, many of the P. aeruginosa strains have acquired multidrug antimicrobial resistance. Exposure to quinolones in vitro or in vivo causes P. aeruginosa from dogs to develop resistance more rapidly than other bacteria.19,141 Pseudomonas fluorescens is able to grow in packed erythrocyte blood units.73a The growth occurs slowly at 4° C and more rapidly at 20° C. To prevent its occurrence, stored blood units must be monitored for discoloration and detection by microscopy and PCR confirmation. For further information on blood product infections, see Chapter 93.
Gram-Negative Bacterial Infections
Etiology
Classification
Antigenic Structure
Epidemiology and Pathogenesis
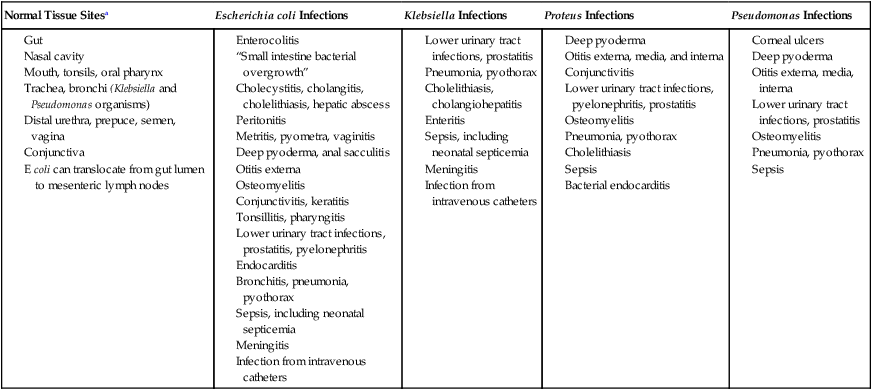
Normal Tissue Sitesa
Escherichia coli Infections
Klebsiella Infections
Proteus Infections
Pseudomonas Infections
Virulence Factors
Virulence Factors Associated with Escherichia coli Intestinal Infections
Classification
Mechanism of Pathogenicity
Clinical Characteristics of Pathogenic Escherichia coli Strains
Enteropathogenic E. coli
Attaching and effacing lesions
Loss of microvilli, watery and chronic diarrhea
Enterohemorrhagic E. coli and E. coli O157:H7
Attaching and effacing lesions, toxins
Hemorrhagic colitis, diarrhea, hemolytic uremic syndrome
Enterotoxigenic E. coli
Toxins
Secretory diarrhea without fever
Necrotoxigenic E. coli
Toxins
Diarrhea, sepsis, and urinary tract infections in people; diarrhea, sepsis, and pneumonia in small animals
Adherent invasive E. coli
Invasion
Granulomatous colitis
Enteroaggregative E. coli
Attachment, toxin
Nonhemorrhagic, watery small bowel diarrhea with some inflammation in humans; not documented in dogs and cats
Enteroinvasive E. coli, Shigella
Invasion
Mucohemorrhagic diarrhea with leukocytes (dysentery)
Extraintestinal pathogenic E. coli or uropathogenic E. coli
Attachment
Urinary tract infections, pneumonia, sepsis, meningitis
Diffusely adherent E. coli
Attachment
Small bowel diarrhea and urinary tract infections in humans, not documented in dogs and cats
Neonatal meningitis E. coli
Attachment and invasion
Neonatal meningitis in humans, not documented in dogs and cats
Cell-detaching E. coli
Diarrhea in children, not documented in dogs and cats
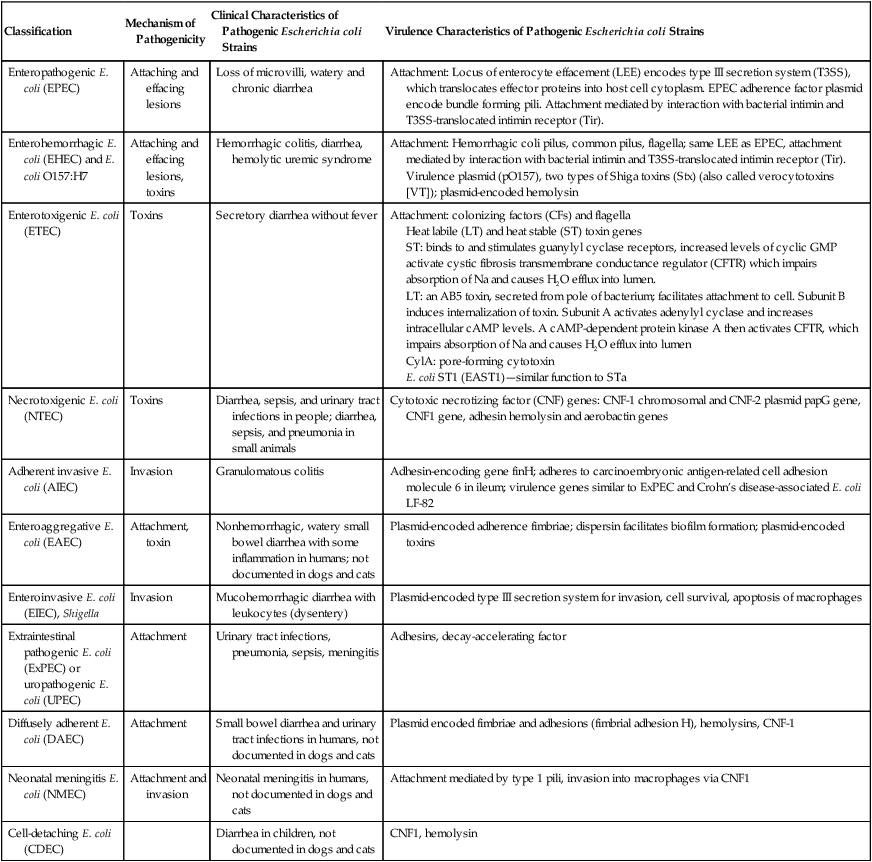
Classification
Mechanism of Pathogenicity
Clinical Characteristics of Pathogenic Escherichia coli Strains
Virulence Characteristics of Pathogenic Escherichia coli Strains
Enteropathogenic E. coli (EPEC)
Attaching and effacing lesions
Loss of microvilli, watery and chronic diarrhea
Attachment: Locus of enterocyte effacement (LEE) encodes type III secretion system (T3SS), which translocates effector proteins into host cell cytoplasm. EPEC adherence factor plasmid encode bundle forming pili. Attachment mediated by interaction with bacterial intimin and T3SS-translocated intimin receptor (Tir).
Enterohemorrhagic E. coli (EHEC) and E. coli O157:H7
Attaching and effacing lesions, toxins
Hemorrhagic colitis, diarrhea, hemolytic uremic syndrome
Attachment: Hemorrhagic coli pilus, common pilus, flagella; same LEE as EPEC, attachment mediated by interaction with bacterial intimin and T3SS-translocated intimin receptor (Tir).
Virulence plasmid (pO157), two types of Shiga toxins (Stx) (also called verocytotoxins [VT]); plasmid-encoded hemolysin
Enterotoxigenic E. coli (ETEC)
Toxins
Secretory diarrhea without fever
Attachment: colonizing factors (CFs) and flagella
Heat labile (LT) and heat stable (ST) toxin genes
ST: binds to and stimulates guanylyl cyclase receptors, increased levels of cyclic GMP activate cystic fibrosis transmembrane conductance regulator (CFTR) which impairs absorption of Na and causes H2O efflux into lumen.
LT: an AB5 toxin, secreted from pole of bacterium; facilitates attachment to cell. Subunit B induces internalization of toxin. Subunit A activates adenylyl cyclase and increases intracellular cAMP levels. A cAMP-dependent protein kinase A then activates CFTR, which impairs absorption of Na and causes H2O efflux into lumen
CylA: pore-forming cytotoxin
E. coli ST1 (EAST1)—similar function to STa
Necrotoxigenic E. coli (NTEC)
Toxins
Diarrhea, sepsis, and urinary tract infections in people; diarrhea, sepsis, and pneumonia in small animals
Cytotoxic necrotizing factor (CNF) genes: CNF-1 chromosomal and CNF-2 plasmid papG gene, CNF1 gene, adhesin hemolysin and aerobactin genes
Adherent invasive E. coli (AIEC)
Invasion
Granulomatous colitis
Adhesin-encoding gene finH; adheres to carcinoembryonic antigen-related cell adhesion molecule 6 in ileum; virulence genes similar to ExPEC and Crohn’s disease-associated E. coli LF-82
Enteroaggregative E. coli (EAEC)
Attachment, toxin
Nonhemorrhagic, watery small bowel diarrhea with some inflammation in humans; not documented in dogs and cats
Plasmid-encoded adherence fimbriae; dispersin facilitates biofilm formation; plasmid-encoded toxins
Enteroinvasive E. coli (EIEC), Shigella
Invasion
Mucohemorrhagic diarrhea with leukocytes (dysentery)
Plasmid-encoded type III secretion system for invasion, cell survival, apoptosis of macrophages
Extraintestinal pathogenic E. coli (ExPEC) or uropathogenic E. coli (UPEC)
Attachment
Urinary tract infections, pneumonia, sepsis, meningitis
Adhesins, decay-accelerating factor
Diffusely adherent E. coli (DAEC)
Attachment
Small bowel diarrhea and urinary tract infections in humans, not documented in dogs and cats
Plasmid encoded fimbriae and adhesions (fimbrial adhesion H), hemolysins, CNF-1
Neonatal meningitis E. coli (NMEC)
Attachment and invasion
Neonatal meningitis in humans, not documented in dogs and cats
Attachment mediated by type 1 pili, invasion into macrophages via CNF1
Cell-detaching E. coli (CDEC)
Diarrhea in children, not documented in dogs and cats
CNF1, hemolysin
Enteropathogenic Escherichia coli
Enterohemorrhagic Escherichia coli and Escherichia coli O157:H7
Enterotoxigenic Escherichia coli
Necrotoxigenic Escherichia coli
Adherent Invasive Escherichia coli
Enteroinvasive Escherichia coli
Extraintestinal Pathogenic Escherichia coli
Uropathogenic Escherichia coli
Klebsiella Infections
Pseudomonas Infections
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Veterian Key
Fastest Veterinary Medicine Insight Engine