CHAPTER 15 Gastrointestinal Function Testing
Patients with GI disease present with signs reflecting compromise to one or more of these functions (Table 15-1). Although our ability to assess GI function is limited, it is sometimes useful to evaluate patients critically from this perspective and also serves as a reminder of the complexity of the GI system.
Table 15-1 Summary of Gastrointestinal Functions and Signs of Compromise
| Function | Signs of Compromise |
|---|---|
| Motility | Anorexia, regurgitation, vomiting, constipation, diarrhea |
| Digestion | Diarrhea, weight loss, polyphagia |
| Absorption | Diarrhea, weight loss |
| Permeability | Diarrhea, weight loss |
| Immunological responses | Anorexia, diarrhea, vomiting, weight loss |
THE ROLE OF GI FUNCTION TESTS
The investigation of cats with GI disease can be challenging because clinical signs often are nonspecific and the initial patient evaluation (Table 15-2) frequently is unremarkable. Therefore it is essential to investigate GI function systematically before considering more invasive diagnostic tests or therapeutic trials. Function tests may provide a definitive answer, but more often the results confirm or localize GI disease and complement other clinical findings (e.g., imaging studies, histopathological findings) (Box 15-1). Ideal function tests have high positive and negative predictive values, good accuracy (defined by both a high sensitivity and high specificity), involve minimal patient risk or discomfort, and have a low cost/benefit ratio. The positive and negative predictive values of a test provide an indication of the reliability of the result (either positive or negative) in a given patient. However, they are difficult to determine and most studies therefore only report the sensitivity and specificity of the test in question. These parameters are inferior to positive and negative predictive values because they do not address the prevalence of disease in a certain patient population. This chapter will review both established and novel GI function tests (Table 15-3) and describe their indications and limitations.
Table 15-2 Minimum Database for a Cat with Suspected Gastrointestinal Disease
| Data | Comments |
|---|---|
| History | Include information on diet and food intake |
| Physical examination | Assess weight loss, body condition, and hydration status |
| Complete blood count | Including blood smear examination |
| Chemistry profile | Including electrolytes |
| Urine analysis | Including sediment examination |
| Thyroid evaluation | Indicated in any cat over 5 years of age |
| Retroviral testing (FeLV and FIV) | Indicated in any cat if >12 months since last test |
| Fecal examination | Flotation and direct examination; PCR test for Tritrichomonas foetus, especially in young cats |
FeLV, Feline leukemia virus; FIV, feline immunodeficiency virus; PCR, polymerase chain reaction.
Table 15-3 Gastrointestinal Function Tests
| Function | Function tests |
|---|---|
| Motility | |
| Digestion | |
| Absorption | |
| Permeability | |
| Immunological responses |
* Barium-impregnated radiopaque markers.
MOTILITY
SECONDARY MOTILITY DISORDERS
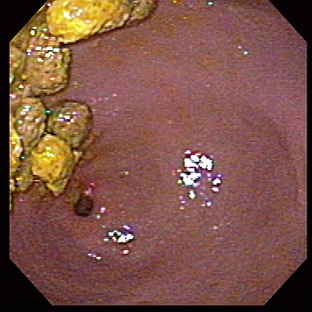
Changes in GI motility can occur in many cats with inflammatory or infiltrative bowel disease. For example, retained food is noted commonly during endoscopy in cats with GI disease and is a reliable indication of delayed gastric emptying (Figure 15-1). The mechanisms behind secondary motility disorders are not well understood, but, most likely, locally produced inflammatory mediators (e.g., cytokines) disrupt the intrinsic enteric nervous system.1 Functional/paralytic ileus (with global impairment of normal contractility) sometimes is noted in cats with severe enteritis, although other causes (e.g., peritonitis, opioid administration) are more common.
ASSESSMENT OF PHARYNGEAL AND ESOPHAGEAL MOTILITY
Fluoroscopic Contrast Studies
Pharyngeal and esophageal motility disorders are best appreciated with fluoroscopic contrast studies, although still images also may provide a diagnosis.2 The patient should be conscious because sedation limits the cat’s ability to protect the airway from aspiration and may affect the swallowing response. In general, 5 mL of a liquid barium sulfate suspension (45 to 85 per cent w/w) should be administered and images collected immediately. If this study is unremarkable, food impregnated with barium should be given and further images collected.
ASSESSMENT OF GASTRIC MOTILITY
Radiographic Contrast Studies
Contrast radiographic studies are a simple and readily available method to assess gastric emptying.3 However, results are influenced by patient anxiety, the use of sedation, and the physical and biochemical properties of the mixture administered. In general, a barium-water slurry usually leaves the stomach within an hour, whereas food generally is cleared by 4 to 12 hours.4 A high fat content may slow gastric emptying substantially, and longer times (up to 16 hours) have been reported for healthy cats. Misleading results can be obtained if the barium separates from the food and leaves the stomach before the ingested material.
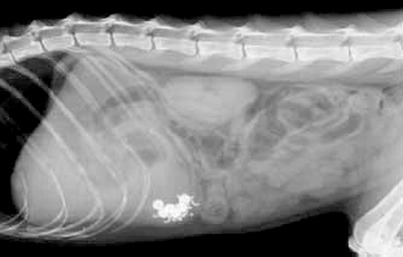
Barium-impregnated radiopaque markers (BIPS, Med I.D. Systems) can be used (Figure 15-2) instead of food mixed with a liquid contrast agent. BIPS also may provide information about transit through the small intestine and colon.5 A mixture of small (1.5-mm diameter) and large (5-mm diameter) spheres is given with specific foods (listed in the package insert), and two to four radiographs are taken over the next 6 to 24 hours. The transit patterns of the two differently sized spheres are thought to mimic liquid-phase and solid-phase gastric emptying. Normal gastric clearance and intestinal transit curves are provided by the manufacturer for comparison. However, gastric emptying times determined using BIPS do not correlate well with scintigraphical studies, and it has been suggested that the 1.5-mm spheres may exceed the threshold size for liquid-phase passage through the feline pylorus.6
Nuclear Scintigraphy
Scintigraphy generally is regarded as the gold standard for assessment of gastric emptying. A radio-labeled marker (e.g., technetium or indium) is mixed with food, and the gastric area is scanned at timed intervals using a gamma camera. The activity measured then is converted into a graph depicting the rate of passage of ingesta from the stomach. If two markers are used, the same test can evaluate emptying times simultaneously for both liquid and solid materials. This test is performed routinely in human beings, and advanced data collection and analysis provides additional information about the frequency and velocity of gastric contractions.7
13C-Octanoic Acid Breath or Blood Tests
These tests are simpler than scintigraphy and avoid the technical complications associated with the use of radioactive materials. Instead, the metabolism of a foodstuff labeled with a stable carbon isotope is used to indicate gastric emptying.8 A 13C-labeled medium chain triglyceride (octanoic acid) is mixed with a test meal, and serial breath or blood samples are collected at regular intervals over the next several hours. After the labeled fatty acid leaves the stomach, it is absorbed immediately in the duodenum and then transported via the portal vein to the liver. Hepatic metabolism releases the 13C, which is oxidized to carbon dioxide (CO2), which is transported to the lungs and excreted subsequently across the alveoli. Thus a rise of 13CO2 in a gas sample extracted from blood, or in a sample of exhaled air, corresponds with duodenal filling. Breath samples can be collected with a face mask or chamber system and stored at room temperature for several months before analysis using fractional mass spectrometry, or with laser or infrared spectroscopy. Various data points, including the lag time, half excretion time, and latency phase then are calculated and compared to established normal ranges.
13C-octanoic acid testing has been described in research cats, but findings in cats with clinical disease have not been reported.9
Ultrasonography
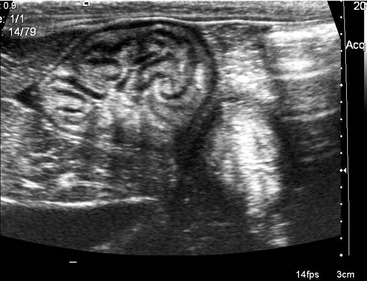
Ultrasonographic assessment of gastric size may provide reliable information about emptying time and has been shown to correlate well with the results of scintigraphy in human beings.10 Serial measurements of a standardized cross-sectional image of the antrum are collected following ingestion of a test meal, until emptying appears complete (Figure 15-3). Operator skill is required for the data to be reliable and consistent, and image collection may be compromised by excessive gas within the gastric lumen.
ASSESSMENT OF SMALL INTESTINAL MOTILITY
Breath Hydrogen Testing
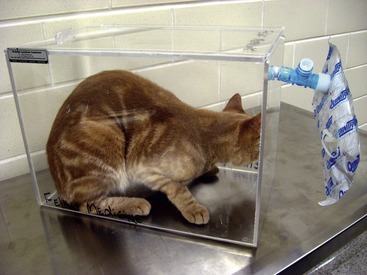
Serial determination of the hydrogen concentration in exhaled air can be used to measure orocecal transit time in cats.11 Test subjects are given meals containing a fermentable sugar (e.g., lactulose), which is metabolized by colonic bacteria. Hydrogen is released as a by-product, enters the blood stream, and is excreted across the lungs. The rise in breath hydrogen concentration therefore indicates the time at which ingested material leaves the ileum. Breath samples are collected using a chamber or face mask (Figure 15-4) and can be stored for several days prior to analysis. Bench-top analyzers are available and provide results in less than a minute. However, no commercial laboratories currently measure hydrogen breath concentrations. Transit times have been shown to be faster in kittens, but findings in cats with signs of GI disease have not been described.12
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree