Chapter 3 Gastrointestinal Diseases
Dental Disease
Ferrets are obligate carnivores with specialized tooth form and function designed to consume animal tissue. Compared with New Zealand feral ferrets, North American pet ferrets have a much greater amount of dental pathology. This is speculated to be a result of dental trauma from inappropriate chewing behavior and kibble-mediated disease.14
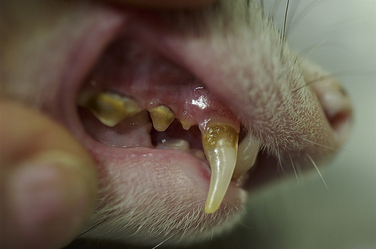
Dry kibble, the mainstay of most pet ferret diets, may be responsible for structural changes to the teeth. These low-moisture, hard crunchy diets appear to be quite abrasive to ferret teeth and result in significant wear to the cheek teeth and molars.14 Although moist or semimoist diets have been associated with the formation of dental calculi and periodontal disease in experimental cases,40 most ferrets on a dry kibble diet develop tartar and gingivitis that progresses with age (Fig. 3-1). Periodontal disease is considered pervasive in pet ferrets (see Chapter 32).
Chewing inappropriate objects (cage bars and toys) can lead to damage. Biting and gnawing habits often result in discoloration, wearing, and breaking of the tips of the canine teeth. Broken canine teeth do not usually result in obvious discomfort or pain unless the dental pulp is exposed. Root canal restoration or surgical removal of the affected teeth may be necessary in some ferrets.44 Tooth root abscesses are uncommon in ferrets. Although dysphagia and drooling are sometimes seen, dental disease is often an incidental finding during physical examination. Dental extractions and scaling can be performed with the animal under anesthesia. Follow the basic principles for dental disease management that apply in the care of the dog or cat. Offering a natural prey diet or moistening the dry kibble may decrease dental abrasions.
Salivary Mucocele
Ferrets have five major pairs of salivary glands: the parotid, submandibular, sublingual, molar, and zygomatic.66 Trauma to a gland can result in extravasation of saliva and salivary mucocele formation. Although this lesion is uncommon in ferrets, mucocele diagnosis and treatment have been described.5,57
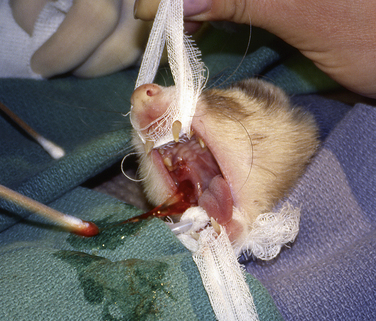
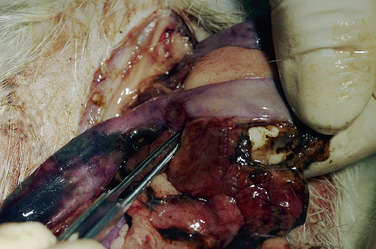
Treatment for salivary mucoceles is usually surgery. In one reported case, scalpel blade lancing of the medial wall of the mucocele resulted in drainage and no recurrence.5 Marsupialization into the mouth with the use of a wide circular incision in the medial wall of the mucocele may be effective for mucoceles that bulge into the oral cavity (Fig. 3-2). Surgical excision of the affected salivary gland is ideal for avoiding recurrence (see Chapter 11). It may be possible to inject contrast medium into the mucocele in an effort to trace the origin of the saliva. Before attempting surgical excision of a salivary gland, review the superficial anatomy of the head and neck region of the ferret.66 Recurrence is possible.
Oral Neoplasia
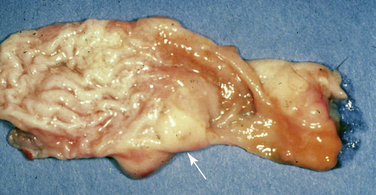
The oral cavity is an uncommon site of neoplasia in ferrets. Squamous cell carcinoma is the most commonly reported oral tumor in ferrets and typically manifests as a firm swelling of the upper or lower mandible.36,38,47 These masses are usually solitary but can appear in situ, in multiple sites (Fig. 3-3).
Treat squamous cell carcinoma with wide surgical excision, including maxillectomy or mandibulectomy as required. In one report, surgical resection of the mass was followed with radiation therapy.36
Esophageal Disease
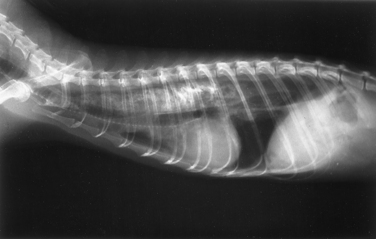
Diseases of the esophagus are rare in ferrets. Acquired megaesophagus has been reported in ferrets8,39 and is occasionally seen in practice. Megaesophagus describes an esophagus that is enlarged (dilated) on radiographic examination and that lacks normal motility. Recognizing this disease is important because the prognosis in ferrets with megaesophagus is poor. Clinical signs include lethargy, inappetence or anorexia, dysphagia, and weight loss. Regurgitation is common. Coughing or choking motions are sometimes described, and some ferrets have labored breathing. Differential diagnoses includes the presence of an esophageal or GI foreign body, gastritis, influenza, and respiratory diseases.
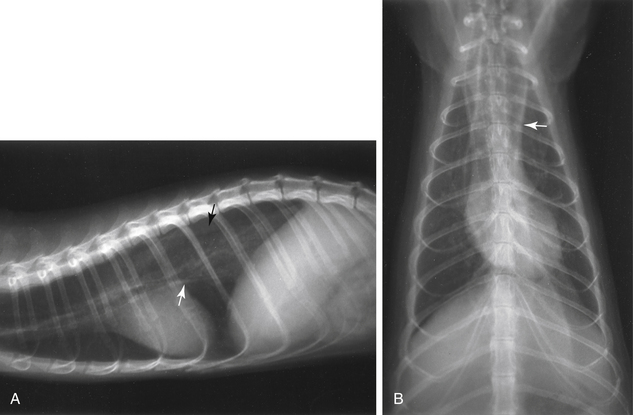
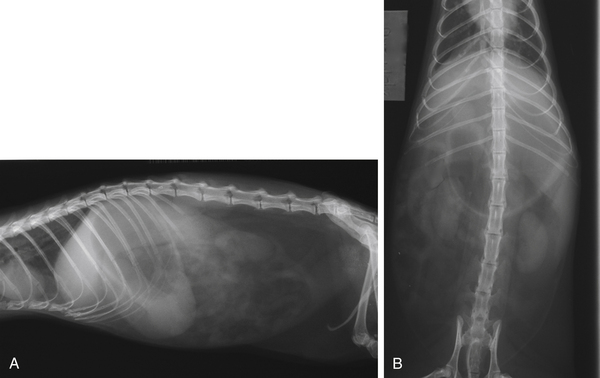
Diagnosis of megaesophagus is based on clinical signs and radiographic evidence. On radiographs, the esophagus is often dilated in both the cervical and thoracic segments (Fig. 3-4). Food may be visualized in the esophagus. Aspiration pneumonia and gastric gas are sometimes evident in addition to esophageal dilation. In suspect cases, always take radiographs of the abdomen to exclude lower GI disease. Administer barium (10 mL/kg by mouth [PO]) to delineate the esophagus and to evaluate mural lesions, strictures, or obstructions (Fig. 3-5). An endoscope can also be used to evaluate the esophagus. Use fluoroscopy, if available, to determine the motility of the esophagus after a barium swallow.
The cause of megaesophagus in ferrets is unknown. Consider possibilities in the differential diagnosis as for dogs, and tailor the diagnostic workup accordingly. To test for myasthenia gravis, serum acetylcholinesterase antibody testing can be performed (Comparative Neuromuscular Laboratory, University of California San Diego, La Jolla, CA; http://vetneuromuscular.ucsd.edu/) and edrophonium chloride (Tensilon) testing is possible, albeit difficult to administer and interpret. Myasthenia gravis has been documented in a young ferret, but an association between megaesophagus and myasthenia was not reported.43
The management of ferrets with megaesophagus is similar to that of canine patients but is less successful. Supportive care and antibiotics are palliative at best. Administration of a GI motility enhancer such as metoclopramide (0.2 to 1 mg/kg PO or subcutaneously [SC] q6-8h) may be helpful. Cisapride, which until recently was marketed for gastroesophageal reflux and gastroparesis in humans, reduces the frequency of regurgitation in dogs with megaesophagus when given at 0.5 mg/kg PO q8-24h.80 This drug has been removed from the market for human use in the United States because of adverse cardiac effects in some people but is available through veterinary compounding pharmacies. Its use in ferrets has not been evaluated. If esophagitis is suspected, add an H2-receptor blocker, such as cimetidine, ranitidine, or famotidine.
Other causes of esophageal disease in ferrets are rare. Esophageal foreign body has been reported in a ferret and was successfully managed surgically.12 One of the authors (HH) has seen a ferret with a sponge foreign body lodged in its distal esophagus; the sponge was broken into smaller pieces by using a 2.7-mm rigid endoscope. The foreign material then passed through the gastrointestinal tract without incident.
Gastritis and Ulceration
Gastric and duodenal ulcers have been reported in laboratory ferrets and are relatively common in pet ferrets (Fig. 3-6). Causes of GI ulceration are foreign body or toxin ingestion, Helicobacter mustelae infection, neoplasia of the intestinal tract, treatment with nonsteroidal anti-inflammatory drugs (NSAIDs), and azotemia caused by renal disease.
The laboratory ferret is used as an animal model for the study of H. pylori infection in humans. Helicobacter mustelae isolated from the gastric mucosa of ferrets shares many molecular and biochemical features of H. pylori. Infection with H. mustelae in ferrets is associated with varying degrees of gastritis, with or without duodenitis, and can result in ulcer formation (see discussion later in this chapter).26
Ulcerogenic drugs such as nonsteroidal and steroidal anti-inflammatory agents can be associated with ulcer formation in many species. It is rare, however, for ferrets to have GI bleeding with corticosteroids, even at dosages as high as 2 to 3 mg/kg/day. Be careful with NSAIDs in ferrets; overdose of anti-inflammatory agents such as ibuprofen (see Chapter 5) can cause ulceration with prolonged or inappropriate use. Severe uremia and associated melena can develop in ferrets with primary renal disease, but this is uncommon.
Basic diagnostic testing includes whole-body radiography and screening blood tests. Fast the ferret for a short time (4 to 6 hours) to facilitate visualization of a gastric foreign body or hairball. The stomach should be empty, and any residual foodlike material may represent ingested hair or other material. The diagnosis of H. mustelae gastritis may be a diagnosis of exclusion of other common disorders, such as the presence of a GI foreign body. Treatment for H. mustelae gastritis is often based on a presumptive diagnosis. Treat gastritis and gastric ulceration with both specific therapy (according to the diagnosis) and supportive care. Hospitalize sick and anorexic ferrets for fluid therapy and parenteral treatment. Antibiotics are indicated for sick ferrets; consider choosing a combination that will target Helicobacter (e.g., amoxicillin and metronidazole in combination with bismuth or proton-pump inhibitor—see Helicobacter treatment later in this chapter). For ferrets that are not vomiting, offer multiple small feedings of a bland, moist diet such as a/d Canine/Feline (Hill’s Pet Nutrition, Inc., Topeka, KS) or a recovery diet formulated for carnivores such as Carnivore Care (Oxbow Pet Products, Murdock, NE) or Emeraid Carnivore (LafeberVet.com, Cornell, IL). Avoid dry, high-fiber foods. For vomiting animals, withhold food for 6 to 8 hours while closely monitoring for any signs of hypoglycemia (older ferrets often have subclinical insulinomas); then, if vomiting has resolved, introduce small, frequent feedings.
Helicobacter Mustelae Gastritis
Helicobacter mustelae is a gram-negative rod morphologically similar to Campylobacter species that requires a microaerobic environment for growth on artificial media. It is antigenically related and biochemically similar to H. pylori, a human pathogen associated with gastritis and ulcers.26 Virtually all North American ferrets are likely to be exposed to H. mustelae as kits, becoming persistently infected at weaning and developing some degree of gastritis.22 Colonization of the antral area of the stomach and pyloric area of the duodenum with H. mustelae is common in domestic ferrets, unless they are specifically treated or hand reared in isolation.17,22 Colonization is accompanied by a specific immune response, but infection persists despite high serum antibody titers.22 Although infection is common, clinical gastritis and ulcers occur relatively infrequently. Severe gastritis may be evident in gastric biopsy samples from ferrets showing no signs of clinical disease.22
The histopathologic lesions of H. mustelae–associated gastritis in ferrets, like H. pylori gastritis in humans, consist of mucus depletion, loss of glands, epithelial hyperplasia, foci of dysplasia, and leukocyte infiltration. The organism can be observed in silver-stained histologic sections of gastric mucosa.26 Severely affected ferrets that die usually have a single large pyloric ulcer or many small ones, and the stomach and intestinal tract contain digested blood and mucus, causing the ingesta to appear very dark. Cultures of H. mustelae from fecal samples are usually difficult to obtain, even when the organism is readily identified histologically or by culture in gastric biopsy samples.
In humans, chronic infection with H. pylori leads to varying clinical and pathologic outcomes, including chronic gastritis, peptic ulcer disease, and gastric adenocarcinoma or mucosa-associated lymphoid tissue (MALT) lymphoma.61 The severity and distribution of the H. pylori–induced inflammation are key determinants of these outcomes.60 Gastritis involving the antrum is associated with excessive acid secretion and a high risk of duodenal ulcer. Gastritis involving the acid-secreting corpus region of the stomach is associated with hypochlorhydria, gastric atrophy, gastric ulcers, and increased risk of gastric cancer.32
As in humans infected with H. pylori, transient hypochlorhydria develops in ferrets approximately 4 weeks after experimental infection.53 This condition probably facilitates fecal-oral transmission as well as recovery of H. mustelae from feces.55 Infection is associated with urease produced in abundance by H. mustelae, which is detectable in gastric biopsy samples and can be used for presumptive diagnosis. Urease production correlates with the degree of colonization and the occurrence of gastritis in biopsy results.22 A urease breath test is available for humans to aid in diagnosis, and a similar test has been used in ferrets under research conditions but is not practical for clinical use.55 Gastric atrophy and hypochlorhydria are associated with the ability of H. mustelae to persistently colonize the stomach; hypochlorhydria due to loss of parietal cells allows non–urease-producing bacteria to colonize the stomach as well.22 Helicobacter species inhibit secretion of acid by parietal cells in vitro, and this mechanism may also contribute to hypochlorhydria.26 In humans, chronic infection with H. pylori is associated with release of cytokines that impair function of enterochromaffin cells, which are neuroendocrine cells in the gastric mucosa that control acid secretion by releasing histamine. The impaired secretory function of these cells may predispose to hypochlorhydria and gastric carcinogenesis. Loss of parietal cells due to chronic inflammation is, however, the primary cause of achlorhydria in humans and ferrets colonized with their respective Helicobacter species.67
Gastrin is a hormone that stimulates gastric acid secretion and is secreted by the G-cells of the gastric antrum. In humans and probably in ferrets, high levels of gastrin may initiate GI mucosal damage and ulceration. Hypergastrinemia is probably a response to the presence of H. mustelae in the antrum or to its associated inflammation. Hypergastrinemia is abolished after antibiotic therapy eradicates the Helicobacter infection.22
In humans, Helicobacter pylori–associated gastritis is a risk factor for gastric adenocarcinoma and gastric lymphoma, and H. pylori has been classified as a Class I carcinogen by the World Health Organization (WHO).22 There is some evidence that this is also the case in ferrets. As H. pylori does in humans,41 infection with H. mustelae in ferrets stimulates cell proliferation in the gastric mucosa.27 Under research conditions, gastric adenocarcinoma developed in aged ferrets that were naturally infected with H. mustelae and treated with a known gastric carcinogen.22 Spontaneously occurring gastric adenocarcinoma has been reported in pet ferrets. Although in some cases H. mustelae was neither cultured from the lesions nor identified histologically,69,76,78 in other cases, silver-stained organisms that were morphologically compatible with H. mustelae were present in the neoplastic tissues.27
Lymphoid follicles are observed in the gastric mucosa of humans colonized with H. pylori91 and in that of ferrets colonized with H. mustelae26 but not in uninfected individuals. In humans this condition may progress to MALT lymphoma. Eradicating H. pylori usually causes early tumors to regress, implicating the infection as the cause of neoplasia.90,91 Gastric MALT lymphoma associated with H. mustelae infection has also been reported in adult ferrets17 (Fig. 3-7). None of the affected ferrets were treated with antibiotics to eradicate H. mustelae either before or during the illness associated with neoplasia.
Clinical Signs and Diagnosis of H. Mustelae Gastritis with Ulcers
Illness may develop in ferrets 12 to 20 weeks of age under conditions of stress caused by a combination of factors, such as rapid growth, dietary changes or inadequacy, and concurrent diseases. Infection is lifelong in untreated ferrets, and the severity of chronic gastritis increases with age.29 In mature ferrets, the disease may become clinically apparent in animals that are stressed by concurrent disease or by surgery for other conditions such as adrenal disease or insulinoma. Ferrets with severe H. mustelae gastritis and ulcers are lethargic and anorexic, and they rapidly become emaciated. Chronic vomiting may occur. Excessive salivation and pawing at the mouth, which are signs of nausea in ferrets, may be evident. Affected ferrets are often moderately to severely dehydrated and may have mild anemia and melena (Fig. 3-8). Black, tarry feces often stains the fur of the tail and perineal region.
Definitive diagnosis of Helicobacter infection is confirmed by histopathologic examination of a gastric mucosal sample obtained by endoscopic or surgical biopsy. Gastric mucosa or fecal samples can be submitted for polymerase chain reaction (PCR)-based analysis (Taconic Rockville, Rockville, MD, www.taconic.com; Veterinary Molecular Diagnostics, Milford, OH, www.vmdlabs.com). Specialized techniques are necessary for culturing the organism, which is not shed consistently in feces of infected ferrets.31
Treatment of H. Mustelae–Associated Gastritis with Ulcers
Treatment for Helicobacter infection in humans usually consists of “triple therapy” consisting of two antibiotics from different classes, such as amoxicillin or metronidazole and clarithromycin, and a proton pump inhibitor. Differing combinations of antibiotics and proton pump inhibitors are used, with varying success. In humans with resistant Helicobacter infections, “quadruple therapy” with an added bismuth compound is often used. Bismuth interferes with the colonization of H. pylori in humans and suppresses colonization of H. mustelae in ferrets.77 Bismuth also has direct antimicrobial actions against Helicobacter.64
In ferrets, the initial treatment for Helicobacter species in ferrets is commonly a triple-therapy combination of amoxicillin, metronidazole, and bismuth subsalicylate, administered q12h for at least 2 weeks (Table 3-1). Oral veterinary or pediatric amoxicillin suspensions are palatable and well accepted by most ferrets; metronidazole can be compounded into a suspension for oral administration. Although H. mustelae is sensitive to either amoxicillin or metronidazole, treatment with one of these antibiotics alone or single treatment with other antibiotics is ineffective for eradication therapy. Other drug combinations have been used in ferrets to eradicate H. mustelae, with advantages of improved palatability and convenience of dosing. Clarithromycin (Biaxin, Abbott Laboratories, North Chicago, IL) (12.5 mg/kg PO q8h) in combination with ranitidine bismuth (24 mg/kg q8h) has been shown to eradicate H. mustelae in ferrets.53 Ranitidine bismuth citrate tablets (not available in the United States) may be crushed and mixed with a palatable liquid or compounded, and clarithromycin is available as a pediatric suspension. Both drugs are administered for 14 to 21 days (dosages are given in Table 3-1). A combination of clarithromycin, metronidazole, and omeprazole or clarithromycin and omeprazole proved more effective than triple therapy with amoxicillin, metronidazole, and omeprazole in eradicating H. mustelae in research ferrets.2 Resistance to clarithromycin has not yet been reported in ferrets but does occur in humans.53 To prevent development of macrolide-resistant strains, clarithromycin should be combined with a second antibiotic not in the macrolide class.2 Chloramphenicol has no effect on H. mustelae.61 Colloidal bismuth subcitrate (8 mg/kg PO q8h) may be substituted for bismuth subsalicylate.
Table 3-1 Summary of Suggested Treatment Regimens for Helicobacter mustelae Gastritis, Inflammatory Bowel Disease, Proliferative Bowel Disease, and Eosinophilic Gastroenteritis
| Disease | Drug | Dosage |
|---|---|---|
| Helicobacter mustelae gastritis | ||
| Original triple therapya | AmoxicillinMetronidazole | 10 mg/kg PO q12h20 mg/kg PO q12h |
| Bismuth subsalicylate | 17 mg/kg (1 mL/kg) PO q12h | |
| Alternative therapya | Clarithromycin | 12.5 mg/kg PO q8h36 |
| Ranitidine bismuth citrate | 24 mg/kg PO q8h36 | |
| or | ||
| Clarithromycin | 50 mg/kg PO q24h1 | |
| Omeprazole | 4 mg/kg PO q24h1 | |
| Metronidazole | 75 mg/kg PO q24h1 | |
| H2 receptor blocker | Famotidine | 0.5-1 mg/kg PO, SC q24h |
| Inflammatory bowel disease | Azathioprine | 0.9 mg/kg PO q24-72h |
| Prednisone | 1 mg/kg PO q24h | |
| Sucralfate | 100 mg/kg PO q6h | |
| Hypoallergenic diet | ||
| Proliferative bowel disease | Chloramphenicol | 50 mg/kg PO, IM, SC q12h |
| Eosinophilic gastroenteritis | Prednisone | 1.25-2.5 mg/kg PO q24h |
| Ivermectin | 0.4 mg/kg SC, PO once; repeat in 14 days |
IM, Intramuscular; PO, per os; SC, subcutaneous.
Chloramphenicol, ranitidine bismuth citrate, azathioprine, and metronidazole can be prepared as suspensions by compounding pharmacists.
a Treat for a minimum of 14 days.
Antacid therapy may not be helpful in the early treatment of Helicobacter infection because affected ferrets usually develop transient hypochlorhydria.31 However, once affected with gastritis and inappetence, antacids can decrease discomfort, improve appetite, and reduce effects of acid reflux on esophageal mucosa. Famotidine (0.50-1.0 mg/kg PO q24h) or other H2-receptor blockers, sucralfate suspension (25 to 100 mg/kg PO q8h), or a proton-pump inhibitor such as omeprazole (4 mg/kg PO q24h) (if not used in triple therapy) may be helpful in very sick animals that are bleeding from extensive gastric ulcers.
Although eradication of H. mustelae is accompanied by decreasing antibody titers, lesions may take longer to resolve.53 Successfully treated ferrets can be reinfected with H. mustelae through contact with infected ferrets.4 For treated ferrets to remain free of H. mustelae, they should not be exposed to ferrets of unknown Helicobacter infection status until the newly introduced ferrets have also been treated.
Gastrointestinal Polyps
Gastric Distention (Bloat)
Pet ferrets occasionally are seen with an acute gastric or small intestinal foreign body blockage that results in a distended, fluid-filled stomach. These ferrets are acutely very weak, reluctant to ambulate, and anorexic. To confirm the diagnosis, take full-body radiographs (Fig. 3-9). These animals are in shock and need immediate aggressive therapy that includes intravenous fluids and decompression. Relieve gastric pressure by placing an orogastric tube (8- or 10-French red rubber feeding tube), treat the hypovolemic shock, and prepare these cases for surgery when stable.
Pyloric stenosis and gastric outflow obstruction can manifest as an acute bloat in the ferret. Pyloric adenocarcinoma in ferrets and in humans has been associated with infection with H. mustelae.69,76 Pyloric stenosis caused by muscular hypertrophy of the pylorus has been seen clinically by one author (HH), with one case occurring in a 4-month-old ferret. Pyloromyotomy and dilation of the pyloric outflow is the recommended treatment, especially where Helicobacter and cancer are not present.
Other than associated with foreign body disease, gastric bloat is rarely seen in pet ferrets, but it has been reported on domestic ferret farms and in black-footed ferrets (Mustela nigripes).21,73 Clinical signs are usually observed in weanling ferrets and include acute gastric distention, dyspnea, and cyanosis. Sudden death can occur. The cause is unknown but is thought to be related to an overgrowth of Clostridium perfringens (previously called C. welchii). Certain conditions may predispose to clostridial overgrowth, including increased concentration of carbohydrates in the GI tract from overeating, dietary changes, and intestinal hypomotility. Clostridium perfringens multiplies rapidly, producing enterotoxins that attack the villous epithelial cells of the gut. Gas production by the bacteria results in abdominal distention.
Gastrointestinal Foreign Bodies
Gastrointestinal foreign bodies are common in ferrets.58,84 Ferrets are naturally inquisitive and like to chew on miscellaneous environmental objects, particularly rubber or sponge products. Rubber or foam foreign bodies are most commonly ingested by young ferrets (younger than 2 years of age); in contrast, trichobezoars (hair balls) are more common in older ferrets (Fig. 3-10). Linear foreign bodies, commonly ingested by cats, are very rare in ferrets.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree