Chapter 15 GASTRINOMA, GLUCAGONOMA, AND OTHER APUDomas
REGULATORY PEPTIDES OF THE GUT
CLASSIFICATION AND DISTRIBUTION OF GUT PEPTIDES.
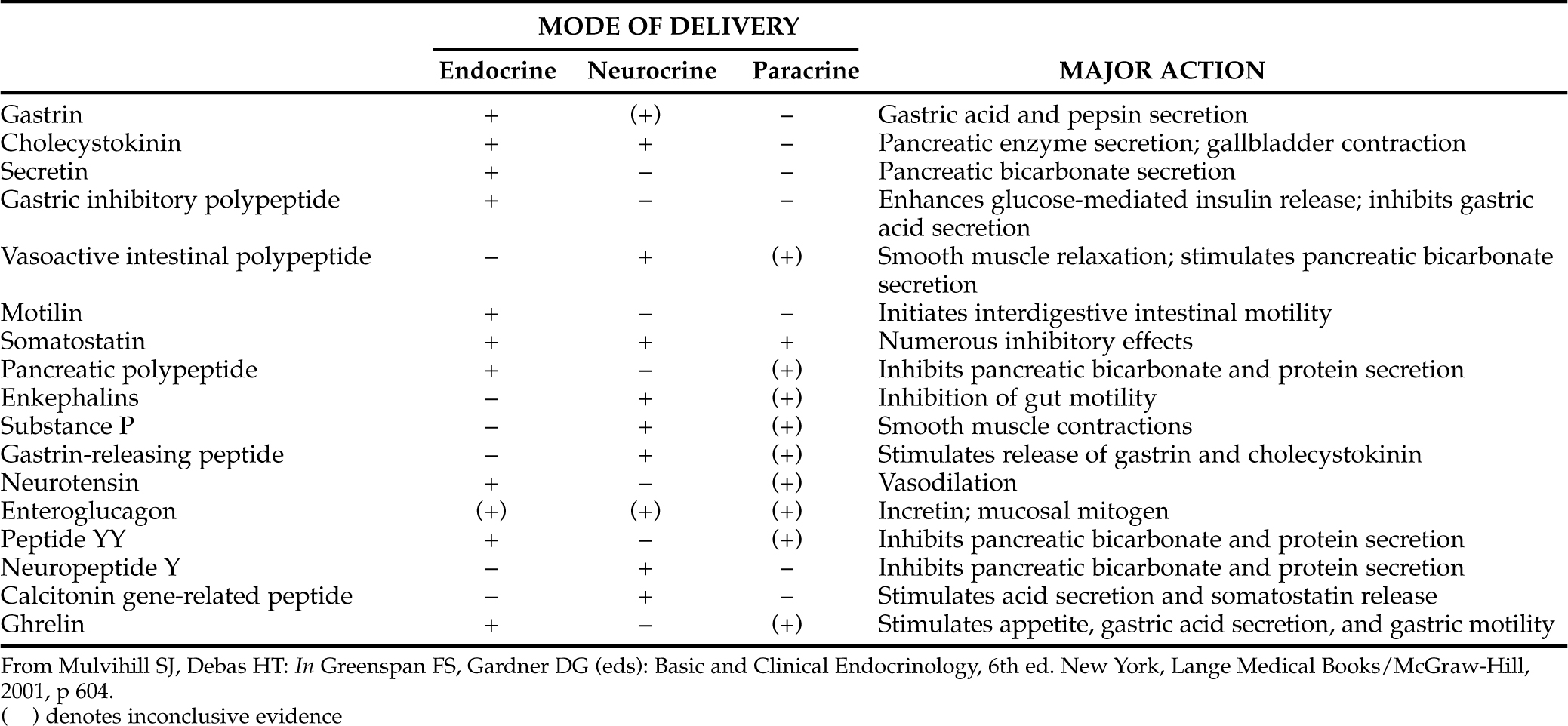
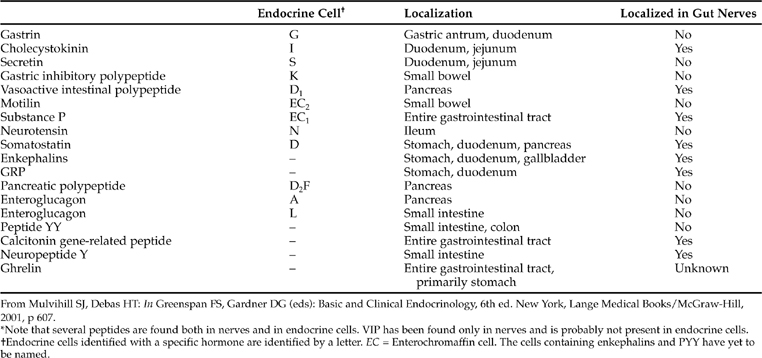
Throughout the gastrointestinal tract, from the esophagus to the rectum, is found a spectrum of specialized endocrine cells that secrete peptides involved with the normal physiologic functions of the gastrointestinal tract (Table 15-1). In addition to specialized endocrine cells, gut peptides are also located in neurons widely dispersed throughout the gastrointestinal tract (Table 15-2). In enteric neurons, gut peptides probably act as neurotransmitters or neurocrine agents (Mulvihill and Debas, 2001). The bodies of enteric neurons are located within the gut wall, usually in the submucosal or myenteric plexus, from which their pathways extend to cells of the mucosa, smooth muscle, blood vessels, and other endocrine cells. This enteric nervous system is considered a division of the autonomic nervous system.
PHYSIOLOGIC ACTIONS.
Gastrointestinal peptides regulate gastrointestinal motility as well as secretion of fluid and digestive enzymes (see Table 15-1). Gastrointestinal peptides also have several trophic actions that regulate metabolism and growth of gastrointestinal tissues by stimulating protein, RNA, and DNA synthesis; minimizing protein catabolism; and enhancing cellular uptake of amino acids (Mulvihill and Debas, 2001). The interactions between these peptides are diverse and complex, encompassing not only stimulation or repression of each other’s secretion but also potentiation or inhibition of each other’s actions on target organs within the gastrointestinal tract. These complex interactions maintain an optimal environment for efficient digestion and absorption of nutrients. As such, gastrointestinal endocrine cells must be capable of monitoring and responding to changes within the environment of the gastrointestinal tract. These responses must occur in a coordinated fashion if optimal assimilation of ingested nutrients is to be maintained.
MODES OF GUT PEPTIDE DELIVERY.
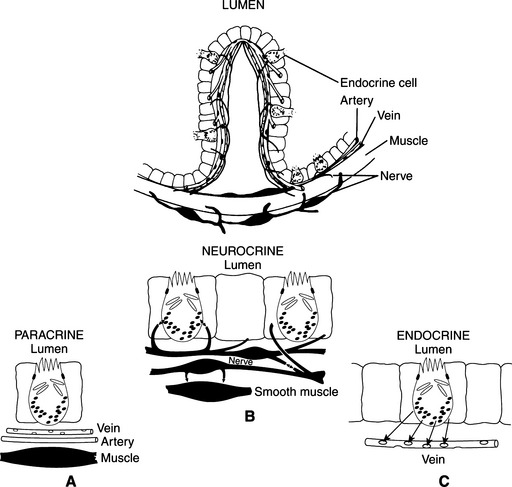
Gastrointestinal peptides are delivered to their sites of action in three main ways: some circulate in the bloodstream in order to reach the target cell (endocrine delivery); some are released into the interstitial fluid and affect nearby cells (paracrine delivery); and still others, within neurons, act as neurotransmitters or neuromodulators (neurocrine delivery; Fig. 15-1; Mulvihill and Debas, 2001). Some peptides have more than one delivery. Because of their various modes of delivery, it is preferable to refer to these substances as regulatory peptides rather than as hormones.
THE APUD CONCEPT
All specialized peptide-secreting cells, including the gastrointestinal endocrine cells, have a number of ultrastructural and cytochemical features in common, some of which reflect the ability of these cells to synthesize and metabolize biogenic amines (i.e., epinephrine, norepinephrine, dopamine, and serotonin). Two of these characteristic features (i.e., amine precursor uptake and decarboxylation) earned the cells the acronym APUD. Most of the cells possessing these characteristics are found in the gut or CNS (hypothalamus, pituitary gland), but they are also present in the thyroid (calcitonin cell), parathyroid, and placenta (Mulvihill and Debas, 2001). Despite their disparate locations, cells that exhibit these properties have important developmental and functional characteristics in common, including the ability to secrete peptide hormones.
An APUDoma is a tumor arising from the APUD cells. This name was first applied by Szijj et al in 1969 to a medullary carcinoma of the thyroid C cells that was secreting ACTH. Since then, several different APUDomas have been described in both the human and the veterinary literature (Table 15-3). APUDomas are named after the endocrine product that they secrete.
TABLE 15-3 CLASSIFICATION OF GASTROINTESTINAL APUDomasAND PRINCIPAL HORMONE SECRETED
| Tumor Type | Principal Hormone Secreted |
|---|---|
| Insulinoma | Insulin |
| Pheochromocytoma | Epinephrine, norepinephrine |
| Gastrinoma | Gastrin |
| VIPoma | Vasoactive intestinal polypeptide |
| Glucagonoma | Glucagon |
| Somatostatinoma | Somatostatin |
| Pancreatic polypeptide-oma | Pancreatic polypeptide |
| Carcinoid syndrome | Serotonin |
The cells composing an APUDoma usually possess the APUD characteristics with even greater clarity than the parent cells. Although it is relatively easy to distinguish one normal APUD cell from another by ultrastructural evaluation of the granules, it is difficult to predict the nature of the polypeptide synthesized or the cell of origin of an APUDoma based on evaluation of the tumor granules. In addition, one tumor may secrete several hormones (Middleton and Watson, 1983).
INSULINOMA
Insulin-secreting islet cell tumors of the pancreas are the most commonly recognized APUDoma in the dog but are rare in the cat. Beta-cell tumors occur most frequently as a single entity. However, they may also represent part of the syndrome of multiple endocrine neoplasia (see Chapter 9). Refer to Chapter 14 for a complete discussion of beta-cell tumors.
PHEOCHROMOCYTOMA
Pheochromocytoma is the second most commonly recognized APUDoma in the dog but is rare in the cat. Pheochromocytoma is a catecholamine-producing tumor derived from chromaffin cells in the adrenal medulla. It occurs most frequently as a single entity but may also be part of the syndrome of multiple endocrine neoplasia. See Chapter 9 for a complete discussion of pheochromocytoma and multiple endocrine neoplasia syndrome.
GASTRINOMA: ZOLLINGER-ELLISON SYNDROME
ETIOLOGY.
In 1955, Zollinger and Ellison described a clinical entity in two human patients consisting of gastric hypersecretion, multiple gastrointestinal ulcers, and a non–beta-cell tumor of the pancreas. It was subsequently shown that these tumors produce gastrin (Mulvihill and Debas, 2001) and the triad of hypergastrinemia, a neuroendocrine tumor, and gastrointestinal ulceration became known as the Zollinger-Ellison syndrome (Jensen and Fraker, 1994). The main actions of gastrin are stimulation of gastric acid secretion and parietal cell growth. Hypergastrinemia induces excessive gastric secretion of hydrochloric acid, which is responsible for the development of esophageal, gastric, and duodenal ulcers, the disruption of intestinal digestive and absorptive functions, and the development of clinical signs. The first reported case of a gastrinoma in veterinary medicine resembling the Zollinger-Ellison syndrome was by Jones et al in 1976. Although sporadic case reports in dogs and cats have since appeared in the literature (Happe et al, 1980; Drazner, 1981; Middleton and Watson, 1983; Breitschwerdt et al, 1986; English et al, 1988; Eng et al, 1992; Brooks and Watson, 1997; Altschul et al, 1997; Green and Gartrell, 1997), this syndrome is still considered a rare entity.
In humans, gastrin-producing tumors are found primarily in the pancreas, proximal duodenum, and gastric antrum, although other sites have been reported (Wolfe et al, 1982; Wolfe and Jensen, 1987). These tumors tend to be small, multiple, slow-growing, and malignant. They cause clinical signs primarily as a result of excessive secretion of gastrin. Occasionally, clinical signs are related to the malignant, space-occupying nature of the multiple metastatic masses. Metastasis is primarily to the liver, lymph nodes, and adjacent omentum. Gastrinomas may also be associated with tumors in other endocrine organs, a condition known as multiple endocrine neoplasia (MEN) syndrome (see Chapter 9) (Pipeleers-Marichal et al, 1990).
Similar behavior patterns of gastrinoma are difficult to assess in dogs and cats, primarily because of the small number of affected animals that have been described to date. Nevertheless, of the 24 dogs and 4 cats with Zollinger-Ellison syndrome either reported in the literature or seen by us, 21 dogs and all cats with gastrinoma had a tumor arising from islet cells in the pancreas, with multiple pancreatic masses identified in 4 animals. The tumor was not identified in the pancreas but was identified in the regional lymph nodes in 2 dogs and at the root of the mesentery in 1 dog (Zerbe and Washabau, 2000; Brooks and Watson, 1997; Green and Gatrell, 1997). Metastatic disease involving the liver, adjacent lymph nodes, spleen, or mesentery was identified in 70% of the cases.
The cell of origin of the pancreatic gastrinoma is disputed. The normal pancreatic islets in the adult contain four cell types: A cells secrete glucagon, B cells secrete insulin, D cells secrete somatostatin, and F cells secrete pancreatic polypeptide. However, gastrin appears to be produced during fetal life by D cells. The most widely accepted current hypothesis is a reversion of D cell function back to fetal function, with subsequent secretion of gastrin instead of somatostatin (Krejs, 1998).
Gastrinomas may secrete other hormones (e.g., insulin, ACTH, pancreatic polypeptide) as well as gastrin, a phenomenon that has been documented in dogs and cats as well as in humans. A canine gastrinoma has been reported that produced gastrin and ACTH, while a gastrinoma in a cat produced gastrin, glucagon, and possibly cholecystokinin (Middleton and Watson, 1983; Feldman and Nelson, 1987). In humans, the clinical syndrome resulting from excessive hormone secretion by one of the cell types in multihormonal neoplasms may not be the result of the predominant cell type in the tumor. In addition, metastatic masses from multihormonal tumors are not necessarily composed of the cell type responsible for the clinical picture.
CLINICAL SIGNS.
Almost all of the clinical signs are a result of severe gastric acid hypersecretion. The most common clinical signs are vomiting, weight loss, anorexia, and diarrhea (Table 15-4). Gastric hyperacidity eventually results in ulcer formation, most commonly involving the stomach and duodenum. Gastrointestinal ulceration was found during gastroduodenoscopy, surgery, or at necropsy in 20 (80%) of 25 dogs and cats with gastrinoma, with perforation of the ulcer identified in 4 animals (Zerbe and Washabau, 2000; Brooks and Watson, 1997; Altschul et al, 1997; Green and Gartrell, 1997). Ulcerations, in turn, may cause vomiting, hematemesis, hematochezia, melena, inappetence, weight loss, depression, and abdominal pain. Esophageal reflux of acid gastric contents may lead to esophagitis and ulceration with worsening inappetence, regurgitation, and weight loss. Diarrhea with malabsorption and steatorrhea may develop following acidification of the intestinal contents, with subsequent inactivation of lipase, precipitation of bile salts, interference with chylomicron formation, and damage to intestinal mucosal cells. Hypergastrinemia may also alter intestinal absorption of water and electrolytes.
TABLE 15-4 FREQUENCY OF OCCURRENCE OF CLINICAL SIGNS IN 24 DOGS AND 3 CATS WITH GASTRINOMA AND THE ZOLLINGER-ELLISON SYNDROME
| Clinical Sign | Percent of Patients |
|---|---|
| Vomiting | 93 |
| Weight loss | 89 |
| Anorexia | 75 |
| Diarrhea | 67 |
| Lethargy, depression | 56 |
| Polydipsia | 22 |
| Melena | 22 |
| Fever | 19 |
| Abdominal pain | 15 |
| Hematemesis | 15 |
| Hematochezia | 7 |
| Tachycardia | 7 |
| Abdominal mass | 4 |
| Polyphagia | 4 |
| Obstipation (alternating with diarrhea) | 4 |
PHYSICAL EXAMINATION.
Findings on the physical examination depend, in part, on the chronicity of the disorder, severity of hyperacidity and ulceration, and presence of a perforated ulcer. Findings of the physical examination can vary from relatively unremarkable to a dog or cat that is extremely sick. Animals with the Zollinger-Ellison syndrome may be lethargic, thin to emaciated, febrile, dehydrated, and in shock. Mucous membranes may appear pale as a result of anemia due to ulcer diathesis. Compensatory tachycardia and abdominal tenderness during palpation may also be present. One cat affected with gastrinoma had a palpable abdominal mass at the time of presentation (Zerbe and Washabau, 2000).
CLINICAL PATHOLOGY.
Abnormalities in the complete blood count that have been associated with the Zollinger-Ellison syndrome in dogs and cats include a neutrophilic leukocytosis, hypoproteinemia, and regenerative anemia, presumably caused by gastrointestinal inflammation and blood loss. Abnormalities identified in the serum biochemistry panel include hypoproteinemia, hypoalbuminemia, hypocalcemia, and mild increases in serum alanine aminotransferase and alkaline phosphatase activities. Hypocalcemia may be a result of concurrent hypoalbuminemia or secondary to gastrin-induced thyroid C-cell hyperplasia and increased calcitonin secretion. Hypokalemia, hypochloremia, and metabolic alkalosis may develop in those dogs and cats with frequent vomiting. Hyperglycemia and hypoglycemia have been reported in a few cases, the cause of which is unknown but may be related to tumor-associated secretion of other hormones (e.g., ACTH, insulin; Zerbe and Washabau, 2000). Results of the urinalysis are usually unremarkable. Evaluation of a fecal sample with Sudan stain may reveal steatorrhea due to both lipid and fatty acid accumulation. A positive occult blood reaction or overt melena is usually present.
DIAGNOSTIC IMAGING.
Survey abdominal radiographs are usually normal. If an ulcer has perforated through the serosal surface, radiographic signs consistent with peritonitis may be present. Contrast radiographic studies may demonstrate gastric or duodenal ulcers; thickening of the gastric rugal folds, pyloric antrum, and/or intestine; and rapid intestinal transit of barium (Zerbe and Washabau, 2000). With concurrent severe esophagitis, a secondary megaesophagus or aberrant, nonperistaltic esophageal motility may be demonstrable fluoroscopically. Potential abnormalities identified with abdominal ultrasound include thickening of the gastric and intestinal wall, gastric ulcers, and a pancreatic mass or its metastases. However, gastrinomas vary tremendously in size and may be microscopic (Roche et al, 1982). Failure to identify a pancreatic mass with ultrasonography does not rule out gastrinoma. Because a high concentration of high affinity somatostatin receptors is present in greater than 90% of tissue from human gastrinomas, the use of radiolabeled somatostatin analogues has enabled successful imaging of many gastrinomas, including previously unsuspected metastases (Schirmer et al, 1995; Gibril et al, 1996). A positive scan can also suggest whether a patient will be likely to benefit from therapy with the long-acting somatostatin analogue octreotide, which decreases gastrin release (Ellison et al, 1986). 111Indium-pentetreotide scintigraphy successfully detected gastrinoma and its metastases in a dog with metastatic gastrinoma, and octreotide treatment was beneficial in decreasing plasma gastrin concentrations (Altschul et al, 1997).
ENDOSCOPY.
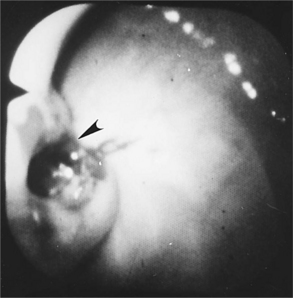
Gastroduodenoscopy in a dog or cat with the Zollinger-Ellison syndrome may reveal severe esophagitis and ulceration, especially in the vicinity of the cardia. Gastric rugal folds may be thickened and persist despite insufflation of the gastric lumen with air. Gastric and duodenal hyperemia, erosions, or ulceration is often visible (Fig. 15-2). Histologic evaluation of esophageal, gastric, and duodenal biopsies obtained during endoscopy may reveal mild to severe inflammation with infiltrates of lymphocytes, plasma cells, neutrophils and/or eosinophils, as well as gastric mucosal hypertrophy, fibrosis, and loss of the mucosal barrier.
DIAGNOSIS.
Measurement of Fasting Serum Gastrin Concentration.
Demonstration of persistent hypergastrinemia in a dog or cat with appropriate clinical signs adds further support for the diagnosis of gastrinoma. Multiple blood samples drawn after an overnight fast provide the most accurate means of establishing hypergastrinemia. Although the normal range for serum gastrin concentration varies among laboratories, the upper limit of the normal range using current radioimmunoassays is typically less than 100 pg/ml in dogs and cats (Altschul et al, 1997; Brooks and Watson, 1997). In dogs with the Zollinger-Ellison syndrome, serum gastrin concentrations have ranged from 72 to 2780 pg/ml, and in two cats it was 350 and 1000 pg/ml. To date, all but one dog and all cats with histologically confirmed gastrinoma had fasting serum gastrin concentrations greater than three times the upper normal value for the laboratory. Fasting serum gastrin concentration was 72 pg/ml (reference range, 10 to 40 pg/ml) in one dog with a 1-month history of vomiting and a neuroendocrine tumor identified at surgery (Green and Gartrell, 1997).
Serum gastrin concentrations may be normal in some humans with proven gastrinoma (Zimmer et al, 1995). Although not yet reported, normal serum gastrin concentrations also seem possible in dogs and cats, especially in the early stages of the disorder. High fasting serum gastrin concentrations are supportive of, but not pathognomonic for gastrinoma. Hypergastrinemia is found with many other disorders, including chronic renal failure, chronic gastritis, gastric outflow obstruction, liver disease, and achlorhydria, and following administration of H2-receptor antagonists (Table 15-5; Breitschwerdt et al, 1986; Breitschwerdt et al, 1991; Goldstein et al, 1998). Fortunately, most of the conditions that cause hypergastrinemia in the dog and cat can be readily differentiated from the Zollinger-Ellison syndrome following evaluation of the initial data base and appropriate diagnostic imaging studies.
TABLE 15-5 FACTORS ASSOCIATED WITH HYPERGASTRINEMIA IN HUMANS AND IN DOGS AND CATS
| Documented in Dogs and Cats | Documented in Humans |
|---|---|
| Gastrinoma | Gastrinoma |
| Renal failure | Renal failure |
| Gastric outflow obstruction | Gastric outflow obstruction |
| Achlorhydria/hypochlorhydria | Achlorhydria/hypochlorhydria |
| Atrophic gastritis | Atrophic gastritis |
| Gastric dilatation/volvulus | Antral gastrin-cell hyperplasia |
| Liver disease | Gastric carcinoma |
| Immunoproliferative | Short bowel syndrome |
| enteropathy of Basenji dogs | Peptic ulcer disease |
| Antacids | Pheochromocytoma |
| H2 receptor antagonists | Vagotomy |
| Proton pump inhibitors | Antacids |
| Glucocorticoids | H2 receptor antagonists |
| Proton pump inhibitors |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree