CHAPTER 5 Fungal Skin Diseases
Cutaneous mycology
General Characteristics of Fungi
The term fungus includes yeasts and molds. The kingdom of Fungi is recognized as one of the five kingdoms of organisms. The other four kingdoms are Monera (bacteria and blue-green algae), Protista (protozoa), Plantae (plants), and Animalia (animals).* Fungi are eukaryotic achlorophyllous organisms that may grow in the form of a yeast (unicellular), a mold (multicellular-filamentous), or both. The cell walls of fungi consist of chitin, chitosan, glucan, and mannan and are used to distinguish the fungi from the Protista. Unlike plants, fungi do not have chlorophyll. The kingdom of Fungi contains five divisions: Chytridomycota, Zygomycota, Basidiomycota, Ascomycota, and Fungi Imperfecti or Deuteromycota.
Fungi have traditionally been identified and classified: (1) by their method of producing conidia and spores; (2) by the size, shape, and color of the conidia; and (3) by the type of hyphae and their macroscopic appearance (e.g., by the color and texture of the colony and sometimes by physiologic characteristics). Therefore, it is important to understand the terms that describe these characteristics. A single vegetative filament of a fungus is a hypha. A number of vegetative filaments are called hyphae, and a mass of hyphae is known as a mycelium. Hyphae are septate, if they have divisions between cells, or sparsely septate, if they have many nuclei within a cell. This latter condition is known as cenocytic. The term conidium (pl. conidia) should be used only for an asexual propagule or unit that gives rise to genetically identical organisms. A conidiophore is a simple or branched mycelium bearing conidia or conidiogenous cells. A conidiogenous cell is any fungal cell that gives rise to a conidium. (Modern taxonomists also may use sexual reproduction characteristics and biochemical and immunologic methods for identification.) There are six major types of conidia: blastoconidia, arthroconidia, annelloconidia, phialoconidia, poroconidia, and aleuriconidia. More detailed information about fungal taxonomy can be found in other texts.*
Characterization of Pathogenic Fungi
Fungi that are pathogenic to plants are distributed throughout all divisions of fungi, but those that are pathogenic to animals are found primarily in the Fungi Imperfecti and the Ascomycota.2,3,6,7
When these interpretational guidelines are not followed, erroneous conclusions can be drawn and reported. For instance, Scopulariopsis brevicaulis was reported to be the cause skin disease in horses in Brazil.25 The organism was seen in skin scrapings and grown in culture. However, hair or tissue invasion was never demonstrated. Neither were therapeutic trials reported. We know that S. brevicaulis is a common, widespread saprophytic fungus that can be isolated from the skin and hair coat of perfectly normal horses (see Table 1-2).
Although gross colonies of dermatophytes are never black, brown, or green, the proper identification of organisms in fungal cultures should be made by medical laboratory clinicians who have expertise in such matters. Detailed information on the cultural growth of four common dermatophytes (Trichophyton equinum, Microsporum gypseum, T. mentagrophytes T. verrucosum) and commonly isolated fungal contaminants is available in other texts (see Chapter 2).*
Normal Fungal Microflora
Horses harbor many saprophytic molds and yeasts on their hair coats and skin (see Table 1-2). The most commonly isolated fungi from horses are species of Alternaria, Aspergillus, Cladosporium, Fusarium, Penicillium, and Scopulariopsis (see Chapter 1).9 Most of these saprophytic isolates probably represent repeated transient contamination by airborne fungi or by fungi in soil.
Dermatophytes are also isolated from the hair coats and skin of normal horses (see Chapter 1).9 It is likely that dermatophytes isolated from normal horses—such as M. gypseum, T. mentagrophytes, T. rubrum, T. terrestre—simply represent recent contamination from the environment. For instance, it is not unheard of to isolate a geophilic dermatophyte, such as M. gypseum, from normal horses or from a horse presented for a skin disease wherein these dermatophytes play no pathogenic role.
Superficial mycoses
Dermatophytosis
Cause and pathogenesis
Trichophyton equinum is the most common cause of dermatophytosis in horses throughout the world.3a,8-10,13–15 Other less frequently isolated dermatophytes include T. mentagrophytes, T. verrucosum, M. equinum, and M. gypseum.8-10,13–15 There is considerable variation in the frequency of isolation of these less common dermatophytes in different parts of the world. Rare causes of equine dermatophytosis include M. canis and Keratinomyces ajelloi.9 Extremely rare isolates include T. tonsurans, T. rubrum, M. nanum, M. audouinii, M. cookei, T. terrestre, T. schoenleinii, M. distortum, and Epidermophyton floccosum.9 Rarely, infections with more than one dermatophyte have been reported.9 Phylogenetic analyses indicate that T. equinum and M. equinum are closely related to the teleomorphs Arthroderma vanbreuseghemii and A. otae, respectively.19
Dermatophytes are transmitted by contact with infected hair and scale or fungal elements on animals, in the environment, or on fomites.9,10 Combs, brushes, clippers, bedding, blankets, tack, fencing, transport vehicles, and other paraphernalia associated with the grooming, movement, and housing of animals are all potential sources of infection and reinfection. The sources of M. equinum or M. canis infections are usually an infected horse or cat, respectively. Trichophyton spp. infections are usually acquired directly or indirectly by exposure to typical reservoir hosts, which may be determined by specific identification of the fungal species or subspecies. For example, most T. mentagrophytes infections are associated with exposure to rodents or their immediate environment. M. gypseum is a geophilic dermatophyte that inhabits rich soil. Infections with anthropophilic species are extremely rare; they are acquired as reverse zoonoses by contact with infected humans. Hair shafts containing infectious arthrospores may remain infectious in the environment for many months to years.
In experimental models of T. equinum or M. gypseum infection in horses,9 the incubation period between inoculation and development of clinical lesions was 6-17 days. Lesions enlarged until 3-10 weeks postinoculation, and then decreased in size and healed by 5-14 weeks postinoculation. With natural infections, incubation periods vary from 1 to 6 weeks.
Cutaneous inflammation is due to toxins produced in the stratum corneum that provoke a sort of biologic contact dermatitis. Host factors are poorly documented, but the host’s ability to mount an inflammatory response plays a critical role in determining the type of clinical lesions produced and in terminating the infection. Dermatophyte infections in healthy horses are usually self-limiting.8-10,13–15 Dermatophytes have shared and specific antigens. T. rubrum and T. mentagrophytes produce substances (especially mannans) that diminish cell-mediated immune responses and indirectly inhibit stratum corneum turnover. These effects could predispose the animal to persistent or recurrent infections. T. equinum and T. mentagrophytes produce urease, gelatinase, protease, hemolysins, and keratinases. In addition, T. equinum produces lipase. These substances can have a variety of proinflammatory and pathologic consequences. Trichophyton spp. can produce proteolytic enzymes that induce keratinocyte acantholysis in vitro and in vivo.31 Dermatophytes have been shown to elaborate penicillinlike substances that can result in the isolation of penicillin-resistant bacteria from affected skin.
Although dermatophytosis may be seen in horses of any age, young animals (less than 2-years-old) are predisposed to acquiring symptomatic dermatophyte infections.8-10,13–15 This is partly due to a delay in development of adequate host immunity. However, differences in biochemical properties of the skin and skin secretions (especially sebum), the growth and replacement of hair, and the physiologic status of the host as related to age may also play a role. Local factors, such as the mechanical barrier of intact skin and the fungistatic activity of sebum caused by its fatty acid content, are deterrents to fungal invasion.
Clinical findings
Dermatophytosis is common in horses.* However, when clinicians rely on clinical signs alone, dermatophytosis (ringworm, tinea) is greatly overdiagnosed. Over a 21 year period, dermatophytosis accounted for 8.9% of the equine dermatology cases seen at the Cornell University Hospital for Animals (CUHA). The analysis of cultures submitted from suspected dermatophytosis cases in horses generally reveals that between 10% and 23.1% are positive.9 Several other dermatoses, especially staphylococcal folliculitis, dermatophilosis, pemphigus foliaceus, and sterile eosinophilic folliculitis mimic the classic ringworm lesion. On the other hand, dermatophytosis is an often missed diagnosis because of the protean nature of the dermatologic findings.
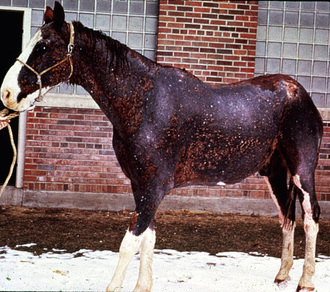
Because the infection is usually follicular in horses, the most consistent clinical sign is one or many circular patches of alopecia with variable scaling and crusting (Fig. 5-1). Some patients may develop the classic ring lesion with central healing and fine follicular papules and crusts at the periphery. However, signs and symptoms are highly variable and depend on the host-fungus interaction and, therefore, the degree of inflammation. Pruritus is usually minimal or absent; however, it is occasionally marked and suggests an ectoparasitism or allergy. In addition, dermatophytosis may be complicated by secondary bacterial (usually staphylococcal) infection. In vitro studies have shown that dermatophytes can produce antibiotic substances and encourage the development of penicillin-resistant staphylococci.
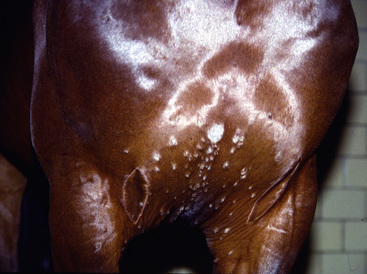
Figure 5-1 Dermatophytosis. Multiple tufted papules and annular areas of alopecia and crusting on brisket.
The initial lesions are often tufted papules, 2-5 mm in diameter. Early lesions may also appear as erect hairs in annular areas of 5-20 mm diameter. Occasionally, an urticarial-like eruption will precede the more obvious follicular dermatosis by 24-72 h. Unlike true urticaria (hives), the lesions do not pit with digital pressure. Hair can easily be plucked from lesions within 4-6 days. Crusts may be thin or thick. Alopecia and a prominent silvery scaling are seen in older lesions. Lesions typically expand peripherally and may coalesce to form polycyclic shapes. Erythema is only seen in white horses. Pruritus is usually minimal to absent and, if present, is most noticeable in the early stages of infection. However, pruritus is occasionally severe and suggestive of an ectoparasitism or an allergy. Variable degrees of pain are often present in early lesions. In horses with acantholytic dermatophytosis or those with secondary bacterial infections, erosions, epidermal collarettes, suppurative exudate, or rare pustules may be present (Figs. 5-2 and 5-3).
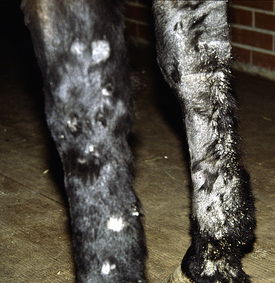
Figure 5-3 Same horse as in Fig. 5-2. Areas of alopecia, scaling, and epidermal collarettes on legs.
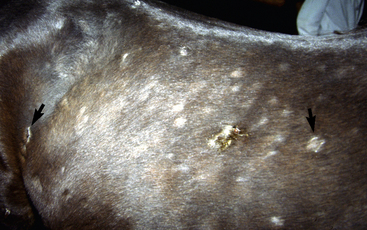
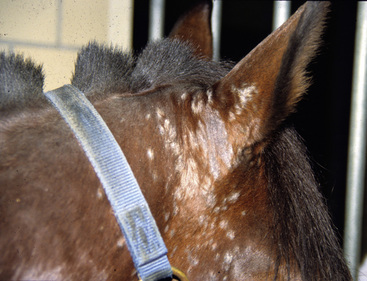
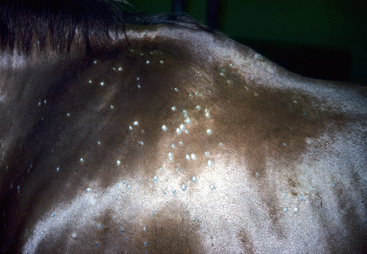
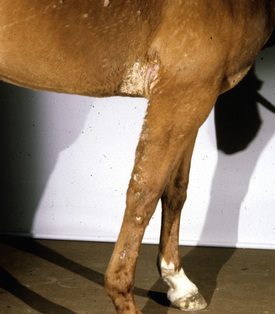
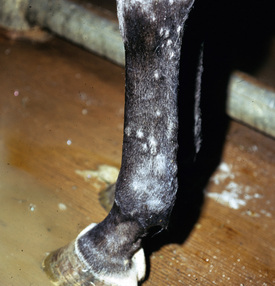
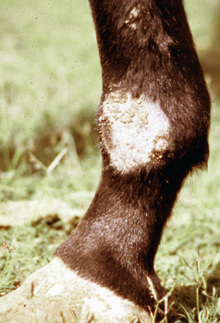
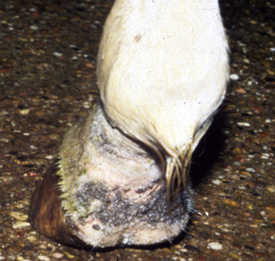
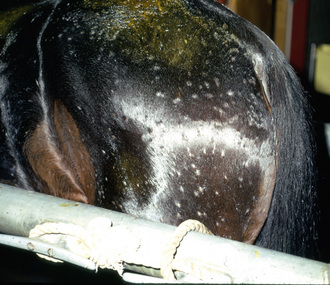
Lesions are most commonly present on the face (Fig. 5-4), neck (Figs. 5-5 and 5-6), dorsolateral thorax (Fig. 5-7), and girth (“girth itch”) (Figs. 5-8 and 5-9). The legs are less commonly affected (Figs. 5-10–5-12). The mane and tail are rarely, if ever, affected. Lesions may be limited to the caudal pastern region (“scratches,” “mud fever,” “grease heel”) (Fig. 5-13), and may wax and wane (analogous to “athlete foot” in humans) with stress, local irritation, moisture, and unsanitary conditions. Lesions are extremely rarely seen on the coronary bands.13–15 Dermatophytosis may also manifest as multifocal to generalized scaling (“seborrhea sicca”) with only irregular ill-defined areas of hair loss, or widespread well-circumscribed alopecia (Fig. 5-14). Rarely, dermatophytic pseudomycetoma is seen in the horse.9,28 It is characterized by one or more subcutaneous to dermal nodules that may be ulcerated and discharging. These nodules are usually present over the dorsal thoracic area and have been caused by T. equinum.
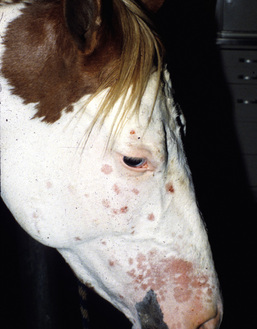
Figure 5-4 Dermatophytosis due to T. equinum. Annular areas of alopecia, scaling, and erythema on the face.
Lesions are usually multiple, and may be very asymmetric or more-or-less symmetric in distribution. Solitary lesions are rarely seen. Generalized dermatophytosis is uncommon and usually seen in immunosuppressed horses or in foals (Figs. 5-14 and 5-15).
In general, the nature of the dermatophyte cannot be determined from the clinical presentation. Some authors have suggested that M. gypseum infections occur most commonly on the face, legs, and dorsal neck and rump (reflecting spread by biting insects), but not on the girth or saddle region.9 Others suggest that M. equinum infections do not involve the girth and saddle regions, and that T. equinum infections rarely affected the head, flank, and rump.9 T. equinum equinum and T. equinum autotrophicum produce identical clinical disease in horses. T. verrucosum infections may produce thicker, gray crusts.9
Dermatophytosis precludes showing, competition, and exportation of affected horses.
Zoonotic aspects
In most areas of the world, dermatophytosis is rarely transmitted from horses to humans. This is because the most commonly isolated equine dermatophyte is T. equinum equinum (see Chapter 2).9,33 Transmission from horse to human is more likely with T. verrucosum9,29,35 and where T. equinum autotrophicum is prevalent (Australia and New Zealand) (see Chapter 2).9 M. canis was reported to be transmitted from a horse to a human. When contracted from horses, human dermatophytosis is characterized by a pruritic, papulopustular (rarely vesicular) dermatitis. Lesions are most commonly seen on the legs (from bareback horse riding) or arms.1,9,32
Diagnosis
Because most infections are follicular, the primary differential diagnoses are staphylococcal folliculitis, dermatophilosis, demodicosis, pemphigus foliaceus, and eosinophilic folliculitis. Demodicosis is extremely rare in horses. Although alopecia areata produces annular areas of alopecia, the alopecic skin appears otherwise normal. Dermatophytic pseudomycetoma must be differentiated from other infectious or foreign-body granulomas, sterile panniculitis, and various neoplasms. When the pastern area is affected, the differential is lengthy (see Chapter 15).
History taking may be of limited value unless exposure is known to have occurred, because clinical dermatophytosis is so variable and the incubation period varies from 6 days to 6 weeks. The number, types, and sources of contact animals should be determined. The degree of contagion is quite variable. Evidence for contagion in other animals or human contacts should be sought. Where horses are grouped together (herds, racing or training centers, and so forth), from 9% to 58% of the animals may be affected.9
Fungal tests are very useful in diagnosis. These tests are described in detail in Chapter 2.
Wood lamp examination for fluorescence causes only certain strains of M. equinum, M. canis, M. audouinii, and M. distortum to produce a positive yellow-green color on infected hairs.9 Because Trichophyton spp and M. gypseum are the commonly isolated fungi, Wood lamp examination is rarely useful in horses. Several important pitfalls exist in the use and interpretation of the Wood lamp (see Chapter 2).
Microscopic examination of plucked hairs may reveal hyphae and arthrospores in 54-64% of the cases, and it is definitive evidence of dermatophytosis (Figs. 5-16 and 5-17) (see Chapter 2).
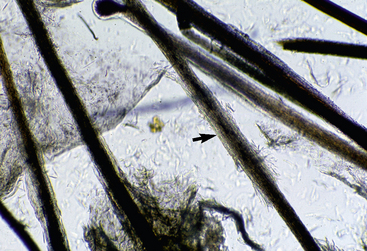
Figure 5-16 Dermatophytosis trichography. Affected hair shaft is focally thickened and fuzzy (arrow).
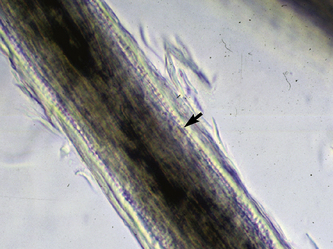
Figure 5-17 Dermatophytosis trichography. Arthroconidia and hyphae are seen within the hair shaft (arrow).
Fungal culture of affected hair and scale is the most reliable diagnostic test and is the only way to identify the specific dermatophyte.8-10,13–15 Caution is warranted here, however, because dermatophytes may be cultured from the hair coat and skin of normal horses and those with nonfungal skin diseases. These dermatophyte isolates may reflect a true carrier state or recent exposure to a contaminated environment. False-positive and false-negative results are possible.9 Cultures may be negative when microscopic examination of hairs is positive. Although dermatophyte test medium is widely used and recommended for culturing dermatophytes, some mycologists have reported it to be unreliable and inferior to Sabouraud dextrose agar.9
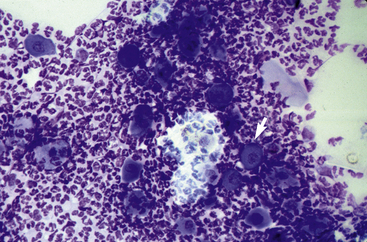
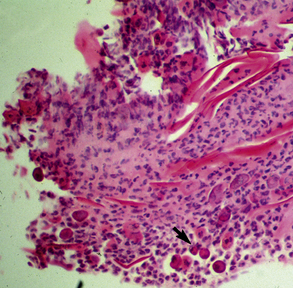
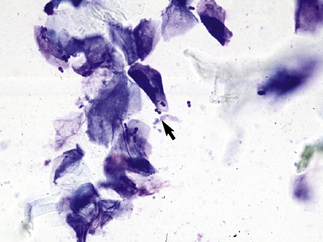
In acantholytic T. equinum infections, cytologic evaluation (direct smears, impression smears) reveals neutrophils and numerous acantholytic keratinocytes, a microscopic image indistinguishable from pemphigus foliaceus (Fig. 5-18).31
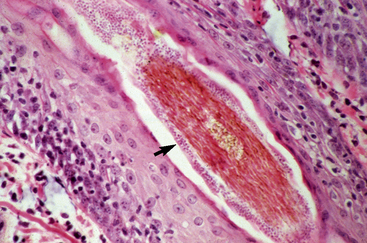
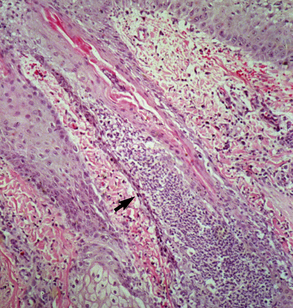
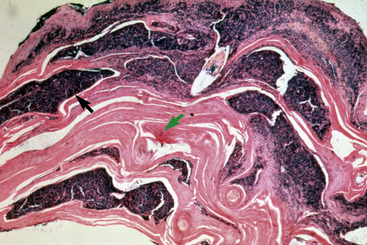
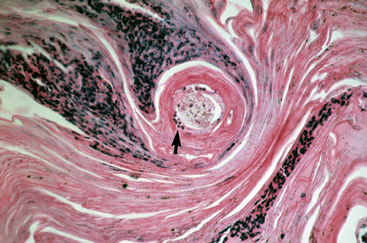
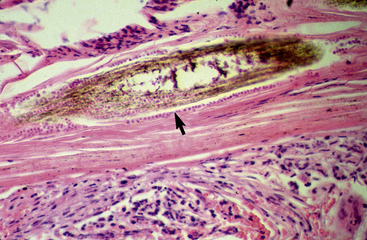
Biopsy findings are as variable as the clinical lesions, and they are not as sensitive as culture.9 On the other hand, when the true significance of a cultural isolation is questioned, demonstration of the organism in biopsy specimens is definitive proof of true infection. The most common histopathologic patterns observed in dermatophytosis are: (1) infiltrative lymphocytic mural folliculitis (Figs. 5-19 and 5-20), suppurative luminal folliculitis (Fig. 5-21), and pyogranulomatous furunculosis (Figs. 5-22 and 5-23); (2) hyperplastic or spongiotic superficial perivascular or interstitial dermatitis with prominent neutrophils and lymphocytes and parakeratotic or orthokeratotic hyperkeratosis of the epidermis and hair follicles; and (3) intraepidermal pustular dermatitis (suppurative, neutrophilic epidermitis).9 Fungal elements are often most easily found in surface scale, crust, and hair fragments. Palisading crusts are identical to those seen in dermatophilosis (Figs. 5-24 and 5-25). Dermatophytic pseudomycetoma is characterized by nodular to diffuse, granulomatous to pyogranulomatous panniculitis and dermatitis wherein the fungus is present as broad (2.5-4.5 μm), hyaline, septate hyphae, chainlike pseudohyphae, and large (12 μm) chlamydospore-like cells within granules (pseudogranules). In horses with T. equinum infection, the reaction pattern can include marked epidermal and/or follicular acantholysis, thus being easily confused with pemphigus foliaceus (Fig. 5-26).31 In such cases, fungal elements may only be present in surface and follicular keratin, not in hair shafts. Septate fungal hyphae and spherical to ovoid arthroconidia may be present in and around infected hairs (Fig. 5-27), in hair follicles, and within the stratum corneum of the surface epidermis. The number of fungal elements present is often inversely proportional to the severity of the inflammatory response.
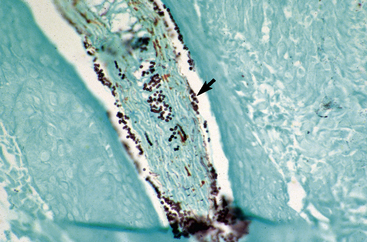
Figure 5-20 Same biopsy specimen as in Fig. 5-19. Special stain reveals numerous arthroconidia and hyphae in hair shaft (arrow) (GMS stain).
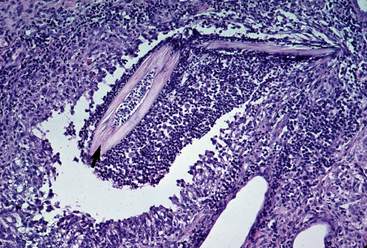
Figure 5-22 Dermatophytosis. Skin biopsy reveals furunculosis and fungal elements in free hair shaft (arrow).
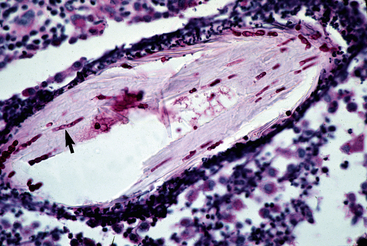
Figure 5-23 Close-up of Fig. 5-22. Special stain reveals fungal elements in hair shaft (arrow) (PAS stain).
Clinical management
Dermatophytosis in healthy horses usually undergoes spontaneous remission within 3 months.4,8-10,13–15 Because of this, a veritable plethora of “therapeutic agents” have been espoused as ringworm cures. Controlled studies documenting the efficacy of this “sea of antifungal agents” in equine dermatophytosis are virtually nonexistent. The goals of therapy are: (1) to maximize the patient’s ability to respond to the dermatophyte infection (by the correction of any nutritional imbalances and concurrent disease states, and by the termination of systemic antiinflammatory and immunosuppressive drugs), (2) to reduce contagion (to the environment, other animals, and humans), and (3) to hasten resolution of the infection. A critical feature of clinical management is the treatment of all horses in contact with the infected horse and treatment of the environment. It is advisable to stop riding, training, or working animals until they are recovered, as continued trauma and exercise may lead to more lesions, worse lesions, and a prolonged recovery. Some authors believe that exposure to sunshine is beneficial.
Topical Therapy
Every confirmed case of dermatophytosis should receive topical therapy. Creams and lotions are available for use on focal lesions, and these are typically applied every 12 h. There is a wide variety of topical antifungals available, and there is no particular advantage of one product over another (Table 5-1). For highly inflamed lesions, a product containing glucocorticoid in combination with antifungal agents may hasten resolution of clinical disease.
TABLE 5-1 Products for the Topical Treatment of Superficial Mycoses
| Product | Indication* | |
|---|---|---|
| Spot Treatment | ||
| Amphotericin B 3% cream, lotion | Fungizone (Apothecin) | C,M |
| Chlorhexidine 4% spray† | ChlorhexiDerm Maximum (DVM) | C,D,M |
| Chlorhexidine 2%/miconazole 2% spray | Malaseb (DVM) | C,D,M |
| Clotrimazole 1% cream, lotion | Lotrimin (Schering) | C,D,M |
| Clotrimazole 1%/betamethasone 0.1% cream | Lotrisone (Schering) | C,D,M |
| Clotrimazole 1%/betamethasone 0.3%/gentamicin ointment | Otomax (Schering) | C,D,M |
| Miconazole 2% cream, 1% lotion, 1% spray | Conofite (Schering) | C,D,M |
| Miconazole 2% spray† | Miconazole Spray (Vétoquinol) | C,D,M |
| Naftifine 1% cream, gel | Naftin (Allergan) | C,D |
| Nystatin cream | Mycostatin (Squibb) | C,M |
| Nystatin/triamcinolone cream, ointment | Animax (Pharmaderm) | C,M |
| Terbinafine 1% cream | Lamisil (Novartis) | C,D |
| Thiabendazole 4% dexamethasone | Tresaderm (Merck Ag Vet) | C,D,M |
| Shampoos | ||
| Chlorhexidine 2% and sulfur 2%† | Seba-Hex (Vétoquinol) | C,M |
| Chlorhexidine 3% | Hexadene (Virbac) | C,M |
| Chlorhexidine 4%† | ChlorhexiDerm Maxi (DVM) | C,M |
| Chlorhexidine 2% and ketoconazole 1% | KetoChlor (Virbac) | C,D,M |
| Chlorhexidine 2% and miconazole 2% | Malaseb (DVM) | C,D,M |
| Miconazole 2% | Dermazole (Virbac) | C,D,M |
| Miconazole 2%† | Miconazole (Vétoquinol) | C,D,M |
| Povidone-iodine | Betadine (Purdue-Frederick) | D |
| Selenium sulfide 1% | Selsun Blue (Chattem) | M |
| Rinses | ||
| Acetic Acid 2.5% | (Vinegar:Water) | C,M |
| Enilconazole 0.2% | Imaverol (Janssen) | C,D,M |
| Lime Sulfur 2-5% | LymDyp (DVM) | C,D,M |
C, candidiasis; D, dermatophytosis; M, Malassezia dermatitis.
* Shampooing may disperse more arthrospores into the hair coat and environment and may not be the most effective nor safe way to treat dermatophytosis.
For horses with multifocal or generalized skin involvement, antifungal rinses (dips) are indicated. Rinses are preferred because the entire body surface can be treated, rubbing of the hair coat is minimized, and the antifungal agent can be allowed to dry on the skin. Rinses are usually applied daily for 5 to 7 days, and then once or twice weekly until clinical cure. Lime sulfur 2-5% (Lym Dyp) and enilconazole 0.2% (Imaverol) are the most effective (see p. 206).4,8-10,13–15 0.2% enilconazole (not approved for horses in the United States) rinses applied once or twice weekly are reported to be effective for the treatment of equine dermatophytosis (see p. 206).9,28,87 Natamycin (100 ppm) (not approved for horses in the United States) spray applied twice weekly is also reported to be effective for the treatment of equine dermatophytosis (see p. 206).22,27,87
Shampoos are less desirable as: (1) they have no residual action, and (2) the physical act of their application and removal may macerate fragile hairs and increase the release and dispersal of infective spores into the coat, thus increasing the likelihood of spreading the infection and of human exposure. Nonetheless, it has been reported that the use of 2% chlorhexidine/2% miconazole shampoo (Malaseb, DVM), twice weekly, resulted in the resolution of clinical signs of dermatophytosis (T. equinum) in horses within 6 weeks (see p. 206).24 Other shampoos used on horses with dermatophytosis include 4% chlorhexidine (ChlorhexiDerm Maximum, DVM), 2% miconazole (Miconazole, Vétoquinol), and povidone-iodine.*
Systemic Therapy
Griseofulvin (Fulvicin U/F) has been recommended for the systemic treatment of equine dermatophytosis.9,10,13–15 Dosage and frequency protocols vary widely. In fact, there are no published data on the pharmacokinetics of griseofulvin in horses, and published clinical trials in equine dermatophytosis are flawed (see p. 207). When oral griseofulvin powder is administered according to the manufacturer’s recommendations, it is of doubtful therapeutic efficacy. Many investigators, including the authors, cannot endorse the use of currently recommended therapeutic protocols for griseofulvin in horses.† See p. 207 concerning the use of griseofulvin.
Ketoconazole (Nizoral, generics), itraconazole (Sporonox, generics), fluconazole (Diflucan, generics), and terbinafine (Lamisil, generics) are effective for the systemic treatment of dermatophytosis in humans, dogs, and cats.9,29 These agents are not presently approved for use in horses in the United States (see p. 207). Ketoconazole is not effective orally (PO) in horses, and itraconazole is prohibitively expensive. Fluconazole would be a logical choice, but no published information is available on its efficacy in equine dermatophytosis.
Vaccination
In Europe, modified live fungal (T. equinum) vaccines were reported to be very effective.9,30,34a The vaccines were administered intramuscularly twice at a 14-day interval. Vaccinated horses developed either no lesions or only a few, short-lived lesions when challenged naturally or experimentally. In the United States, an experimental inactivated (killed) fungal (T. equinum) vaccine was also given intramuscularly twice at a 10- to 14-day interval.26 Eighty-seven percent of the vaccinated horses did not develop dermatophytosis under natural conditions, while 52% of the nonvaccinated horses did develop disease. Over a 3-year period, the vaccine protocol reduced the frequency of equine dermatophytosis from 40% to 0%. In Europe, a polyvalent (T. equinum, T. verrucosum, T. mentagrophytes, M. canis, M. gypseum) vaccine was reported to result in “significantly faster healing” when compared with placebo.20 In Turkey, a lyophilized T. verrucosum vaccine was reported to be superior to placebo for the treatment of naturally-occurring T. equinum infections.34 In Russia, a vaccine (content not specified) was reported to have a 98.7% prophylactic and 96.3% therapeutic efficacy in equine dermatophytosis (no details given).21 There is currently no vaccine available for the prevention or treatment of equine dermatophytosis in the United States.
Environmental Decontamination
Candidiasis
Cause and pathogenesis
Candida spp.(especially C. albicans) yeasts are normal inhabitants of the alimentary, upper respiratory, and genital mucosa of mammals.* Candida species cause opportunistic infections of skin, mucocutaneous areas, and external ear canal. Factors that upset the normal endogenous microflora (prolonged antibiotic therapy) or disrupt normal cutaneous or mucosal barriers (maceration, burns, indwelling catheters) provide a pathway for Candida spp. to enter the body. Once in the body, further spread of infection correlates with cell-mediated immunocompetence and neutrophil function. Immunosuppressive disease states or immunosuppressive drug therapy predispose some animals to candidiasis. Vulvovaginal candidiasis was reported in horses following the PO administration of altrenogest (synthetic progestogen).37 Candida spp. produce acid proteinases and keratinases (degrade stratum corneum), and phospholipases (penetration of tissues). Candidiasis has been reported under the following names in earlier literature: candidosis, moniliasis, and thrush.
Clinical findings
Candidiasis is an extremely rarely reported disease in horses. A 5-year-old Freiberg mare had multiple cutaneous nodules (especially over neck, shoulder, and thorax) that were firm, painful, and covered by normal skin and hair coat.38 The horse also had ventral edema, mastitis, and pyrexia. Histopathologic findings in skin biopsy specimens include nodular granulomatous dermatitis with numerous yeasts (2-6 μm diameter) and blastoconidia (budding yeast cells) within macrophages and multinucleated histocytic giant cells. Culture and fluorescent antibody testing were positive for Candida guilliermondii. Treatment with sodium iodide was curative.
Oral candidiasis (white, pseudo-membranous plaques and ulcers on tongue and gingiva) was reported in immunodeficient foals.36 Mucocutaneous candidiasis may occur in immunologically compromised horses (Fig. 5-28).
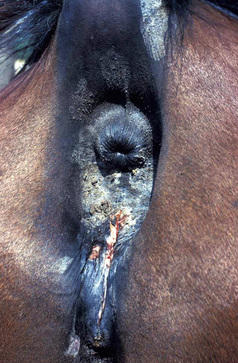
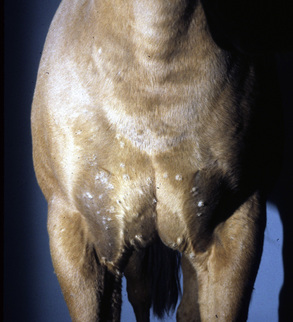
Figure 5-28 Candidiasis. Erythema, ulceration, and crusting in perineal area.
(Courtesy I. Yeruham.)
Vulvovaginal candidiasis was reported in six thoroughbred mares following the PO administration of altrenogest.37 The perivulvar and perineal skin was erythematous, pustular, eroded, and variably hyper- or hypopigmented.
Diagnosis
The differential diagnosis for nodular forms of candidiasis includes infectious and sterile granulomatous disorders. Mucocutaneous candidiasis must be differentiated from immunologic diseases (e.g., pemphigus vulgaris, bullous pemphigoid, systemic lupus erythematosus, erythema multiforme, vasculitis), adverse cutaneous drug reactions, and epidermolysis bullosa. Cytologic examination of direct smears reveals suppurative inflammation and numerous yeasts (2-6 μm in diameter) and blastoconidia (budding cells).2,3,6,7 Pseudohyphae may occasionally be seen. In contrast to Malassezia spp., Candida spp. show narrow-based and multilateral budding. Candida species grow on Sabouraud dextrose agar at 25-30 °C. The API 20C system is a convenient and reliable system for identification.
Clinical management
Correction of predisposing causes is fundamental. Excessive moisture must be avoided. For localized lesions, clipping, drying, and topical antifungal agents are usually effective. Useful topical agents include nystatin (100,000 Unit/gm), azoles (2% miconazole, 1% clotrimazole), 3% amphotericin B, gentian violet (1:10,000 in 10% alcohol), and potassium permanganate (1:3000 in water).9,10 These agents should be applied two to three times daily until lesions are completely healed (1-4 weeks).
Oral, widespread mucocutaneous, and generalized lesions require systemic antifungal therapy. Vulvovaginal candidiasis in mares was cured by the intravaginal insertion of 500 mg clotrimazole per day for 5 consecutive days.37 Intravenous (IV) amphotericin B or ketoconazole, itraconazole, or fluconazole administered PO are effective in other species. These agents have not been evaluated in equine candidiasis. Therapy should be continued for 7-10 days beyond clinical cure (2-4 weeks). One horse with nodular cutaneous candidiasis was cured with the administration of sodium iodide (see p. 208).38
Malassezia Dermatitis
Cause and pathogenesis
One nonlipid-dependent (Malassezia pachydermatis) and seven lipid-dependent (M. equina, M. furfur, M. globosa, M. obtusa, M. restricta, M. slooffiae, and M. sympodialis) Malassezia spp. yeasts have been isolated from normal and abnormal equine skin.9,39-41,45 More than one Malassezia sp. may be isolated from individual horses. Morphological characteristics are unreliable for differentiation of Malassezia spp., and genetic analyses are required. In the United Kingdom, a “new” Malassezia sp. was isolated from normal horse skin, and tentatively named “M. equi.”42,45 However, recent genetic analyses resulted in the official designation of M. equina.40
Many predisposing factors have been hypothesized to allow the commensal Malassezia yeasts to become pathogenic.* Increased humidity is probably important, as Malassezia dermatitis seems to be more common in humid climates (e.g., summer) and in certain anatomic locations (e.g., skin folds). Increased availability of yeast nutrients and growth factors are also probably important: hormonal alterations of the quantity and quality of sebum, “seborrheic” skin (keratinization disorders), and increased populations of commensal symbiotic staphylococci. Immunologic dysfunction, especially as it concerns cell-mediated immunity, could play a role in the pathogenesis of Malassezia infection. Chronic glucocorticoid therapy and immunosuppressive disease may be risk factors. Hypersensitivity to Malassezia antigens likely plays an important role in many horses.
Clinical findings
Malassezia dermatitis is rarely reported in horses, but may be more common than publications would indicate.4,9,43,45 Malassezia dermatitis has most often been recognized in horses with pruritic, greasy-to-waxy, occasionally foul-smelling dermatoses in intertriginous areas: axillae, groin, udder, and prepuce (Fig. 5-29).9,13-15,45 Mares with Malassezia dermatitis in the intermammary fossa rubbed their tails and ventral abdomens. Other horses had facial involvement, possibly associated with heat, humidity, and the occasional trauma provided by face masks.4
Diagnosis
Malassezia dermatitis should be considered a factor in any scaly, erythematous, greasy to waxy, pruritic dermatitis in which other differentials have been eliminated by diagnostic tests and there is a lack of response to treatment (e.g., glucocorticoids, antibiotics, antiseborrheic shampoos, insecticides, miticides).4,9
The most useful and readily available tool for the clinician presented with a suspected case of Malassezia dermatitis is cytologic examination. Samples of surface scale or grease are gathered by making a superficial skin scraping, vigorously rubbing a cotton swab on the skin surface, pressing a piece of clear cellophane tape onto lesional skin several times, or pressing a section of a clean glass microscope slide on the skin. It is not clear which of these methods is the best, and each has its own benefits and shortcomings. Superficial scrapings are reliable, but can be difficult to perform in certain areas. Tape strips are good where the skin surface is flat and not overly waxy or greasy. Direct impression with a glass slide is good for flat surfaces and where grease and wax are plentiful. Cotton swab (Q-Tip) smears are good for skin folds. Whichever method is used, all material is transferred to a glass slide and stained for cytologic examination. One looks for round to oval to the classic peanut-shaped yeasts. Malassezia spp. are characterized by monopolar budding of daughter cells from one site on the cell wall, formation of a prominent bud scar or collar at the site of daughter cell development, a peanut-shape, and a diameter of 3-8 μm. Yeasts are often seen in clusters or adhered to keratinocytes (Fig. 5-30).
The full diagnostic value of cytological examination in horses remains to be determined. In one study of 12 normal horses, yeast were found in one or more cutaneous sites (especially the groin) in seven horses (from less than 1 to over 10 yeast/1000X high power field (HPF)). In another study, samples were taken from the intermammary or prepucial fossa in 11 normal horses (five mares, six geldings) and subjected to cytologic examination and culture.45 Forty of 44 (91%) cytologic evaluations were positive for yeasts. At present, the number of yeasts per microscopic field that differentiates normal horses from those with Malassezia dermatitis has not determined.
Malassezia pachydermatis is usually easy to culture. As it is not lipid-dependent, it grows well on routine Sabouraud dextrose agar at 32-37 °C. However, some strains of M. pachydermatis do show poor growth on unsupplemented media. An atmosphere containing 5-10% carbon dioxide significantly increased the frequency of isolation and colony counts on Sabouraud dextrose agar, but not on modified Dixon agar. The lipid-dependent Malassezia spp. will not grow on Sabouraud dextrose agar, and require alternative, supplemented media. Modified Dixon agar will grow all Malassezia spp. The presumed M. equina isolated from horses did not grow on Sabouraud dextrose or modified Dixon agar, but growth was obtained on Sabouraud dextrose agar enriched with oleic acid and incubated at 30 °C.42 As Malassezia spp. are commensal organisms, their isolation in culture is of little or no practical diagnostic value. In the study wherein intermammary and prepucial fossae of normal horses were sampled for cytology and were cultured, Malassezia spp. were isolated in culture from only seven of 44 (16%) samples.45
In a recent study, four horses with normal skin were sampled from the axilla, groin (intermammary or prepucial fossa), tail base, and perianal area for cytology, culture, and DNA sequencing.44 Yeasts morphologically consistent with Malassezia spp. were seen in every sample, but Malassezia spp. were never cultured. Instead, DNA sequencing showed that all isolates were other saprophytic yeasts: Candida, Myxozyma, Pichia, Dipodascopsis, and Kluyveromyces. Hence, cytologic examination cannot diagnose Malassezia dermatitis.
The histopathologic findings of equine Malassezia dermatitis have not been published. The authors have seen biopsy specimens from two horses with yeast dermatitis (culture and DNA sequencing were not performed). Major histopathologic changes included marked parakeratotic-to-orthokeratotic hyperkeratosis, epidermal hyperplasia, moderate spongiosis, lymphocytic exocytosis, and a superficial interstitial infiltrate of predominantly lymphocytes, plasma cells, and macrophages (Fig. 5-31). Numerous yeasts were present in the surface keratin (Fig. 5-32). Ultimately, the diagnosis of Malassezia (or yeast) dermatitis rests on the response to antiyeast treatment.*
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree