Ryan A. Ferris
Fungal Endometritis
Approximately 1% to 5% of all cases of infectious endometritis are caused by fungal organisms, but fungal endometritis is a clinically important cause of infertility in the mare despite being relatively uncommon. The most commonly cultured organisms in mares with fungal endometritis are Candida spp, Aspergillus spp, and Mucor spp.
History and Physical Examination
Mares predisposed to developing fungal endometritis may have abnormalities of the reproductive tract (e.g., perineal defects, cervical defects, pneumovagina, urovagina, or decreased uterine fluid clearance) or generalized immunosuppression such as is observed with pituitary pars intermedia dysfunction. The use of intrauterine antimicrobials has also been suggested as a cause of increased incidence of fungal endometritis, either through uterine contamination with fungal organisms from repeated infusions or by alteration of the normal bacterial flora of the caudal reproductive tract and facilitation of fungal colonization of the vaginal vault, clitoral fossa, and clitoral sinuses. Colonization of these sites can serve as a nidus for subsequent infection of the uterus.
Diagnosis
A crucial factor in the development of an effective therapeutic plan for fungal endometritis is accurate identification of the etiologic agent. Currently, the standard techniques for detection of fungal endometritis are mycologic culture, microscopic evaluation of endometrial cytology or biopsy samples, and detection of fungal DNA by polymerase chain reaction (PCR) analysis.
Culture
Because of the long growth phase of fungal organisms in culture, mycologic culture often involves a long waiting period for results, and considerable laboratory expertise is necessary for accurate identification. Specialized media such as Sabouraud’s agar should be used to potentiate growth of fungal organisms. Chromogenic agar is available and can tentatively identify and differentiate among some Candida species. Chromogenic agar is ideal for quickly identifying Candida krusei, which has a relatively high mean inhibitory concentration for azole-type antimicrobial agents. Culture of the clitoral sinus and fossa may be warranted to identify a nidus of infection in cases of fungal endometritis that are refractory to treatment.
Cytology
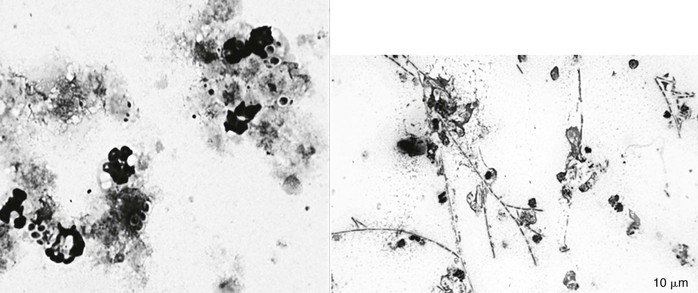
Cytology samples may be obtained by either traditional double-guarded uterine swab or cytology brush or by low-volume uterine lavage. Mares with fungal endometritis usually, but not always, have inflammatory cells in a uterine cytology sample. Fungal organisms are often easiest to detect on smears made from the pellet after centrifugation of uterine effluent recovered from a low-volume lavage. Yeast organisms are typically 5 to 8 µm in diameter with a distinct cell wall (100 to 200 nm) that forms a clear “halo” around the organism. The halo is especially evident when the organism is surrounded by debris or other cells. Hyphate fungi are typically 3 to 5 µm in width and 8 µm in length. These individual cells are commonly linked together in long branching chains (Figure 164-1). In many instances, detection of a fungal organism on endometrial cytology may be the only evidence of fungal endometritis, as organisms do not always grow on culture.
Quantitative Polymerase Chain Reaction
Diagnosis of mycologic infections by in vitro amplification and detection of fungal DNA using molecular techniques (i.e., PCR) is now available in some areas. Molecular assays have the advantage of being more sensitive than other assays, and results are more rapidly available than with culture. Rapid identification of the fungal organisms responsible for the infection allows for early institution of antimicrobial therapy, which could potentially improve the clinical outcome. For instance, Candida albicans is typically sensitive to fluconazole, whereas C krusei and Candida glabrata are inherently resistant to fluconazole. Unfortunately, quantitative PCR assay cannot be used to determine antimicrobial susceptibility for fungal organisms.
Treatment
Treatment for fungal endometritis involves physical removal of infectious organisms by uterine lavage with dilute acetic acid or dilute povidone-iodine, plus systemic or intrauterine infusion of antifungal agents and correction of predisposing factors (poor perineal conformation, urine pooling, or cervical defects) that could result in treatment failure. Administration of more than one antifungal agent may be indicated in refractory or recurrent clinical cases. Uterine lavage is indicated to remove retained fluid, reduce organism load, kill fungal organisms, and remove biofilm. It may also be beneficial to apply topical antifungal medication to the vagina and clitoris because these areas act as a reservoir or nidus for reinfection.
Ideally, selection of an antifungal agent would be based on results of antimicrobial susceptibility tests for each case of fungal endometritis. Unfortunately, antifungal susceptibility tests are not performed in many veterinary diagnostic laboratories, and several weeks often pass from the time of sample submission to when results are obtained. Clinicians often rely on empirical choices for initial antifungal therapy drugs based on published susceptibility patterns while awaiting organism identification and susceptibility testing (Table 164-1).
TABLE 164-1
Susceptibility of Fungi to Drugs Commonly Used to Treat Fungal Endometritis in Mares
| Antifungal Agent | SUSCEPTIBILITY PATTERN (% OF ISOLATES) | ||
| Susceptible | Intermediate | Resistant | |
| Polyenes | |||
| Amphotericin B | 96 | 0 | 4 |
| Natamycin | 100 | 0 | 0 |
| Nystatin | 100 | 0 | 0 |
| Azoles | |||
| Clotrimazole | 80 | 13 | 7 |
| Ketoconazole | 81 | 15 | 4 |
| Miconazole | 43 | 41 | 16 |
| Itraconazole | 62 | 38 | 0 |
| Fluconazole | 44 | 14 | 42 |
| Flurocytosin | 83 | 0 | 17 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree