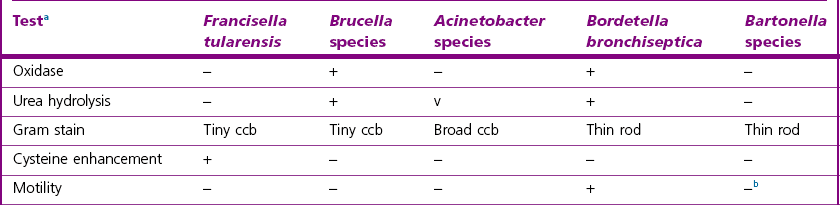
Chapter 22 Fransicella species are tiny, pleomorphic, non-motile, Gram-negative coccobacilli. The genus Francisella can be distinguished from other genera of small Gram-negative coccobacilli using the features outlined in Table 22.1. The genus Francisella is within the Francisellaceae family and consists of F. tularensis, F. noatunensis, F. hispaniensis, F. halioticida and F. philomiragia. Francisella species are strict aerobes that grow best on blood agar supplemented with cystine or chocolate agar. They are oxidase-negative (except F. philomiragia), weakly catalase-positive and non-spore forming. They also have a limited range of carbohydrates fermentation (acid without gas) and a unique fatty acid composition. Table 22.1 Presumptive identification of Francisella tularensis and differentiation from similar Gram-negative genera of significance in veterinary medicine a+ = greater than or equal to 90% positive, − = less than or equal to 10% positive, v = variable, 11 to 89% positive; ccb, coccobacilli; Francisella tularensis is widely distributed in nature. However, it occurs mainly in the northern hemisphere and most frequently in Scandinavia, North America, Japan and Russia. It has also been reported from Turkey, Yugoslavia, Spain, Kosovo and Switzerland indicating a wider distribution (Ellis et al. 2002). Francisella tularensis subsp. tularensis (type A) is the most virulent strain and is found in North America, while F. tularensis subsp. holarctica (type B) is less virulent and is found in North America, Europe and Asia. Three biovars of F. tularensis subspecies holarctica have been suggested; biovar I (erythromycin sensitive), biovar II (erythromycin resistant) and biovar japonica (Kudelina & Olsufiev 1980, Olsufjev and Meshcheryakova 1982). Francisella tularensis subsp. mediasiatica has only been isolated from Central Asia and is considered to be of low virulence. Strains of the subspecies novicida cause severe disease in inbred mice, similar to F. tularensis subsp. tularensis human isolates, but are not pathogenic for immunocompetent humans (Baker et al. 1985). Francisella tularensis has been isolated from about 250 wildlife species that can potentially transmit tularaemia to humans. Wild animals are thought to be reservoirs of infection, especially rabbits and hares but also beavers, muskrats, squirrels, woodchucks, opossums, skunks, deer, voles, foxes, rats and other rodents (Ellis et al. 2002, Zhang et al. 2006). The bacterium is most frequently transmitted by any one of a large range of biting arthropods including flies (Chrysops and Tabanus), mites, mosquitoes (Culicidae), lice and ticks (Ixodes, Dermacentor and Amblyomma). These arthropod vectors probably play a role in the mechanical transmission of the disease both between wild animals and from animals to humans. It is also thought that the tick may serve as a reservoir of infection (Foley & Nieto 2010). Rural populations and especially individuals who spend time in endemic areas such as hunters, agricultural and forestry workers have a higher risk of contracting tularaemia (Ellis et al. 2002). Francisella tularensis is quite resistant in the environment and can survive for months in soil, water, on carcasses and meat. It is susceptible to common disinfectants (70% ethanol, sodium hypochlorite, glutaraldehyde) and autoclaving. Francisella tularensis can infect a wide range of species of wild and domestic mammals, birds, fish, reptiles and amphibians. Tularaemia is acquired by direct contact with infected animals, through contaminated water or food, or from vectors such as biting insects or ticks. Airborne transmission also occurs, especially during processing of agricultural products. The disease is often epidemic, both in humans and in animals. Clinical manifestations depend on the type of reservoir involved and the means of transmission. An appreciable number of infections in farm animals can occur in some regions. Tularaemia is rarely seen in dogs which are considered relatively resistant. However, cats are more susceptible and there are reports of transmission from cats to humans. In animals, tularaemia has a large spectrum of clinical signs ranging from no signs of illness to death. Characteristic signs include depression, anorexia, fever, vomiting, diarrhoea, lymphadenomegaly, ulcers and haemorrhage. Human tularaemia can occur in several forms (ulceroglandular, glandular, oropharyngeal or gastrointestinal, oculoglandular, pneumonic and typhoidal), largely depending on the route of entry of the organism into the body. The most common form is the ulceroglandular disease that occurs as a consequence of an infected arthropod vector bite (Ohara et al. 1998, Ellis et al. 2002). In some cases, infection occurs via cuts or abrasions, typically in hunters after the handling of contaminated meat. An ulcer will then form at the site of infection and flu-like symptoms follow after an incubation period of three to six days. Although F. tularensis is one of the most infectious bacterial pathogens, its virulence mechanisms (Table 22.2) are only beginning to be understood (Meibom & Charbit 2010). The organism resides within host macrophages in vivo. The capsule of Francisella, although essential for serum resistance, is not required for survival following phagocytosis by leukocytes (Sandstrom et al. 1988). Phase variation of the lipopolysaccharide (LPS) of F. tularensis has been reported and the different forms appear to affect both antigenicity (due to variations in the O antigen) and the nitric oxide (NO) response of macrophages (due to variation in the lipid A moiety). Phase variation of lipid A also affects the ability of the organism to grow intracellularly. One phase (reduced NO induction) results in bacterial growth, while another phase (increased NO production) suppresses growth, thus modulating the innate immune response (Cowley et al. 1996). In addition, the LPS of F. tularensis does not show the properties of a classical endotoxin. For example, it fails to induce interleukin-1 from mononuclear cells and poorly induces tumour necrosis factor. A Francisella pathogenicity island (FPI of 30 Kb) has been discovered (Nano et al. 2004) and several virulence-associated proteins identified (Santic et al. 2005, Brotcke et al. 2006, Lenco et al. 2007), in addition to a putative type IV secretion system, encoded by the FPI. Table 22.2 Main virulence factors of pathogenic Francisella tularensis To survive and evade phagosome–lysosome fusion, intracellular pathogens have evolved different mechanisms such as escaping from the phagosome into the cytoplasm after degradation of the phagosomal membrane or adapting to the acidic environment within the phagolysosomes. However, the more common strategy is to modulate phagosome biogenesis to faciliate intracellular replication. Francisella tularensis has a specific mechanism of intracellular development with a unique method of modulating phagosome biogenesis. Studies have shown that F. tularensis enters human macrophages by a novel process of engulfment within asymmetric, spacious pseudopod loops, a process that differs from all previously described uptake mechanisms (Clemens et al. 2005). The Francisella-containing phagosomes have limited maturation in the endocytic pathway and do not fuse with lysosomes (Clemens et al. 2004). Within hours the organism disrupts the phagosome and escapes into the cytoplasm where it replicates. Escape from the cell follows cell disruption due to pyroptosis, a form of apoptosis induced by the organism (Foley & Nieto 2010).
Francisella tularensis
Genus Characteristics
Natural Habitat
Pathogenesis and Pathogenicity
Virulence determinants
Functions
AcpA protein
Acid phosphatase function, capable of inhibiting the respiratory burst
Capsule
Essential for serum resistance
LPS
Phase variation of LPS appears to affect antigenicity and the nitric oxide response of macrophages
Francisella pathogenicity island (FPI of 30 Kb)
Evasion of lysosomal fusion within macrophages
PdpA and PdpD
Required for intracellular replication in macrophages and for virulence in mice
iglABCD operon
Transcribed in response to iron limitation
IglC and IglA
Necessary for full virulence of F. tularensis
IglA and IglB
Thought to be involved in protein secretion
IglC
Required for growth in macrophages
mglAB operon
Likely a global transcriptional regulator which regulates the expression of a range of proteins in response to nutritional stress
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


