CHAPTER 5 Fertilization
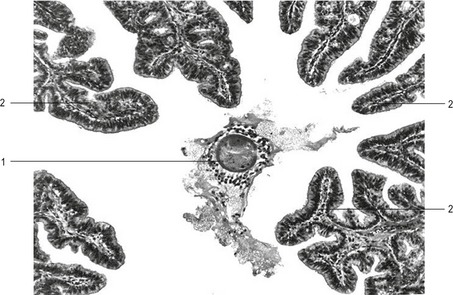
Sexual reproduction occurs through fertilization, during which two haploid gametes fuse to produce a genetically unique individual. Fertilization, the process by which the spermatozoon and the egg unite, occurs in the ampullary region of the oviduct (see Chapter 3). The mammalian egg complex, which is ovulated and enters the oviduct via the infundibulum, consists of three components: (1) the oocyte, arrested at metaphase of meiosis II in most domestic mammals (with the exception of dogs, where the final maturation to metaphase II occurs in the oviduct), (2) the zona pellucida, an extracellular matrix surrounding the oocyte, consisting of glycoproteins that are synthesized by both the oocyte and the surrounding cumulus cells in domestic animals, and (3) the cumulus cells, consisting of several layers of cells from the cumulus oophorus embedded in an extracellular matrix, composed mainly of hyaluronic acid. It has become common to consider the zona pellucida and the oocyte as one, leading the ovulated egg complex to be described as the cumulus-oocyte complex (COC) particularly in the context of biotechnological procedures (Fig. 5-1).
SPERM TRANSPORT IN THE FEMALE GENITAL TRACT
The duration of copulation varies among different domestic animals (see Chapter 3). It takes less than a minute for ruminants to copulate, somewhat longer in horses, several minutes in pigs, and in dogs five to 30 minutes may be necessary. In several mammalian species (cows, sheep, rabbit, dog, cat and primates) the semen is deposited into the cranial vagina. In others (pigs, horses and camelids) the semen is either ejaculated directly into the cervix (pig) or, via the processus urethralis, into both the cervix and uterus.
In the dog, the first of the three fractions of the ejaculate originates from the prostate, the only accessory sex gland in this species. The volume of this clear and acellular so-called presperm fraction ranges from 0.5 to 5 ml, depending on the breed. The second fraction, opalescent in colour, is rich in spermatozoa. Its volume varies between 1 and 4 ml and it contains between 300 million and two billion spermatozoa. The last fraction is also contributed by the prostate. Its volume may vary over a broad range, from 1 to even 80 ml, depending on the breed. Ejaculated in surges, this last fraction of prostatic fluid may force the sperm-rich fraction cranially into the uterus. In the cat the ejaculate volume is small (0.2 to 0.3 ml) and it is unclear whether it comprises multiple fractions.
Retrograde loss of spermatozoa from the female genital tract depends on several factors. The more important are volume and physical nature of the ejaculate and the site of its deposition within the female genital tract. As already mentioned, in some species, like the pig, proteins from the seminal plasma form a conspicuous vaginal plug that prevents spermatozoa from being lost to the exterior. In some laboratory rodents the solid vaginal plug that is formed after copulation can be seen from outside and is used to determine the time of mating (see Chapter 20).
A major barrier for spermatozoal transport is the cervix uteri that can also serve as a reservoir of spermatozoa in several species. In ruminants, and to a lesser degree in the mare, the cervix possesses a convoluted system of folds and grooves. As in other domestic species, the epithelium of the ruminant cervix produces a highly viscous mucus that prohibits the penetration of spermatozoa through the cervical canal during most times of the oestrous cycle. It is only during oestrus that the mucus changes its viscosity, when a low viscosity mucus rich in sialomucins is produced in the basal regions of the cervical crypts. A second type of mucus, containing mainly sulphomucins and much more viscous, is secreted by the apical portions of the epithelium covering the tips of the cervical folds. These two different types of secretion create two different compartments within the cervical canal, one basal with low viscosity and one more central with high viscosity. The low viscosity environment in the basal regions of the folds offers a ‘privileged pathway’ through which spermatozoa can quite easily move towards the uterus. The ability of spermatozoa to use this privileged pathway depends on their ability to swim through the crypts of the cervix; non-motile spermatozoa cannot progress and are eliminated. Consequently, the cervix acts as a filter for removing non-viable spermatozoa.
CAPACITATION
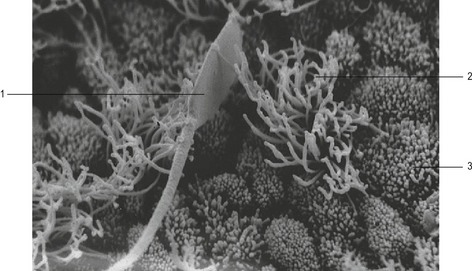
Spermatozoa are not able to fertilize the oocyte immediately after arrival in the female genital tract; to acquire fertility they must reside there for a certain period of time (Fig. 5-2). The changes that occur during this period constitute capacitation of spermatozoa. The site where capacitation takes place varies to some extent among species. In species in which spermatozoa are delivered into the mid-cervix (sow), or into the caudal cervix and immediately enter the corpus uteri, capacitation probably begins in the uterus and ends in the isthmus of the oviduct. In species with intravaginal semen deposition, capacitation is probably initiated during the passage of the spermatozoa through the cervix. Not all spermatozoa are capacitated at the same time and, as the process usually extends over several hours, individual spermatozoa can show a different degree of capacitation depending on their location within the female genital tract.
Capacitation encompasses a number of complex processes. It has been clearly shown that the plasma membrane of the spermatozoon (particularly of the head) undergoes marked changes during capacitation. Important processes during capacitation are: removal of the glycoprotein coat and seminal plasma proteins (adsorbed during epididymal storage and ejaculation) from the spermatozoal surface; the functional coupling of the signal-transducing pathways that regulate the initiation of the acrosome reaction by the zona pellucida glycoproteins; alterations in the flagellar motility that are necessary to penetrate the zona pellucida; and, finally, development of the capacity to fuse with the plasma membrane of the oocyte. These processes are accompanied by changes in metabolism, the biophysical properties of the plasma membrane, and protein phosphorylation, together with elevation of intracellular calcium levels and pH, and hyperpolarization of membrane potential.
INTERACTIONS BETWEEN SPERMATOZOA AND THE ZONA PELLUCIDA
Adhesion of spermatozoa to zona pellucida
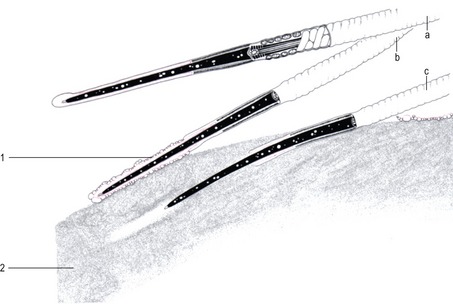
The first contact between the spermatozoon and the ZP, adhesion, is a loose, non-specific association between the gametes and appears to be a disconcertingly random affair (Fig. 5-3). It is followed by a relatively firm binding, which is species-specific and mediated by complementary receptors on the ZP (sperm receptor) and on the sperm surface. In the mouse initial sperm-zona adhesion is mediated by ZP3, a constituent glycoprotein of the ZP that binds to receptors on the anterior head of acrosome-intact sperm. This adhesion to the ZP is probably based on protein-carbohydrate recognition processes, through the association of O-linked α-galactosyl residues from ZP3 with a cognate receptor on the sperm. Secondary binding is then mediated by ZP2. Other authors, however, consider that sperm-zona binding is a purely protein-based event.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree