Chapter 66 Female Elephant Reproduction
Through a combination of different approaches, such as postmortem structure analysis, biochemical investigations, application of imaging techniques, and behavioral observations, considerable advances have been made over the last 4 decades. This has assisted with the markedly improved breeding success of captive Asian and African elephants, as well as the management of their wild populations. The most recent accomplishments include successful manual semen collection, sorting, and freezing, as well as artificial insemination with fresh, chilled, or frozen semen.9
Female Reproductive Tract
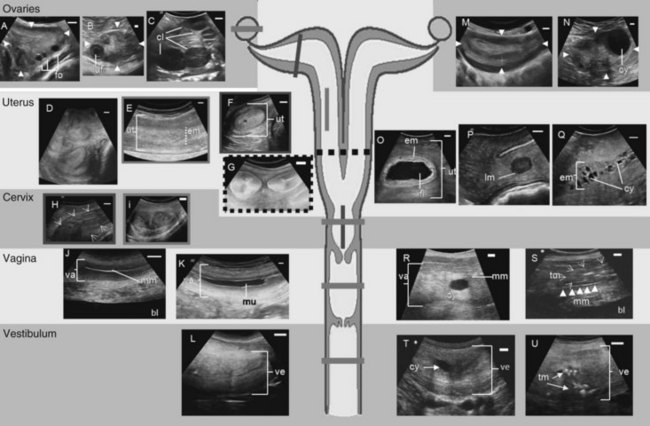
The special anatomy of the elephant’s reproductive organs is regarded as an adaptation to the previous aquatic life of their former ancestors.10 In addition to the size of the entire female urogenital tract (Fig. 66-1), with a total length of about 3 m,14 the most obvious difference compared with other ungulate species is the extremely long vestibule (urogenital canal), which measures about 1.0 to 1.4 m and opens between the hind legs.1,15,26 During urination and relaxation, the large clitoris becomes evident, protruding from the vestibule opening. The tubelike vestibule runs vertically and bends horizontally over the caudal pelvis (see Fig. 66-1L). At its cranial end, a hymen-like structure is found in nulliparous females, leaving only a small opening of approximately 0.4 × 0.2 cm into the vagina. For successful artificial insemination, this tiny vaginal os has to be penetrated with the insemination catheter to deposit the semen into the vagina. This true opening is flanked by two blind pouches. The whole hymenal structure will rupture during the first delivery, but not during mating, because the male elephant delivers his semen into the cranial part of the vestibule. After the first birth, the opening to the vagina is approximately 2 to 3 cm in diameter. The vagina is characterized by longitudinal folds and extends 30 to 50 cm cranially (see Fig. 66-1J, K). The cervix also shows longitudinal folds and is only approximately 15 cm long (see Fig. 66-1H, I). The portio cervices, as the caudal part of the cervix, prominently protrudes into the vagina. The uterus bicornis (see Fig. 66-1G) measures 0.8 to 1.5 m, including a short uterine body of only 5 to 10 cm. The endometrial thickness of the uterine body is different from the horns. The uterine body has a thinner endometrial lining, which may explain why implantation never occurs in this part. However, both body and horns have a similar uterine lining, which shows longitudinal rugae and a ciliated mucous membrane. Each pregnancy damages the endometrium where the placenta has been attached, leaving permanent scars. The comparably small ovaries measure 7.0 × 5.5 × 2.5 cm and weigh approximately 60 g. They have a typical brainlike structured surface and are completely enveloped in a double-pouch formation. The inner serosal pouch forms the oviductal infundibulum. The outer pouch also encapsulates the ovary and their fat inclusions explain difficulties in imaging ovarian structures via ultrasound. In mature healthy females, the ovaries always contain functional structures (see Fig. 66-1A-C), except during late lactational anestrus. Most interesting is the occurrence of multiple corpora lutea (CLs) (up to 42; average, 4 to 6) of sizes between 10 and 50 mm, which protrude from the ovarian cortex in cycling as well as in pregnant cows.
Estrous Cycle
In the wild, female elephants reach maturity at about 10 to 12 years. However, in captivity, puberty may be reached as early as 3.5 to 5 years.16,25 The spontaneous ovulating, polyestric female is usually considered nonseasonal, with births occurring throughout the year, but peaks in the rainy season have been observed in their range countries. In captive herds, natural estrous synchronization has been observed,29 but may rather be an artificial condition because free-ranging elephant females spend most of their reproductive lifespan either pregnant or in lactational anestrus.
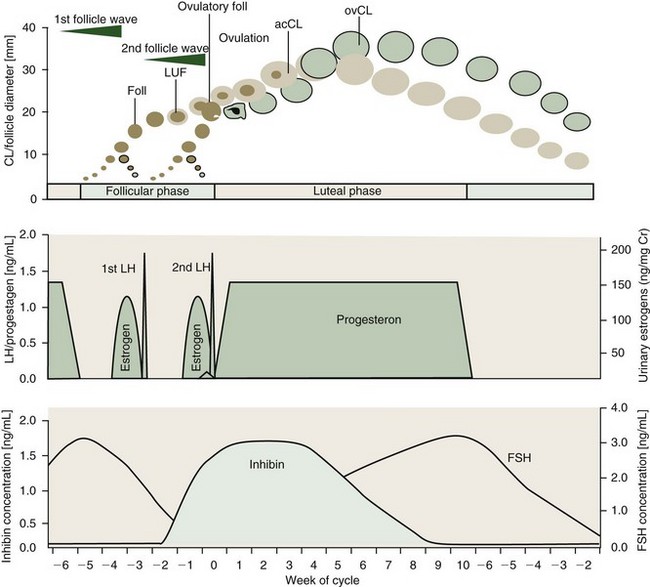
With a duration of 13 to 18 weeks, elephants exhibit the longest spontaneous estrous cycle of any mammal studied to date.3,4 Seasonal differences in the length of the follicular and luteal phases may occur in Asian elephants in their natural environment.27 Estrous cycle activity may be assessed by weekly progestagen determination from serum, urine, or feces. The typical cycle pattern shows a biphasic profile of interchanging periods of elevated (luteal phase, 6 to 12 weeks) and baseline (interluteal or follicular phase, 4 to 6 weeks) progestagen concentrations (Fig. 66-2).3
The most striking difference compared with other mammals is the occurrence of two distinct luteinizing hormone (LH) peaks, during the follicular phase of which only the second induces ovulation (see Fig. 66-2). These two peaks are 19 to 22 days apart and each one is preceded by an estrogen surge.3,5 Each LH peak terminates a follicular wave, but only the second (ovulatory) LH peak will cause a dominant follicle of about 20.0 mm (range, 15.0 to 23.0 mm) to rupture 12 to 24 hours later.11,15,21 The first (anovulatory) LH peak, however, has been shown to induce luteinization of larger follicles of the first follicle wave.21 Luteinized follicles (LUFs) become ultrasonographically apparent about 10 days after the first LH peak. This is coincident with a sharp rise in the inhibin level. Luteal cells of the elephant’s CLs appear to produce inhibin and might be a source of this hormone in addition to the dominant follicle.19,22 Inhibin is known to be an important factor for the selection of the dominant follicle. Therefore, the first LH peak may be to induce luteinization of granulosa cells in larger follicles from the first wave and subsequently secrete inhibins, which in turn promotes deviation of a single dominant follicle at the end of the second follicular wave.22 At the same time, the LUFs are the source of accessory corpora lutea.11,21 There are approximately 2 to 10 of these accessory luteal structures on each ovary of cycling and pregnant elephants, in addition to a corpus luteum (CL) from ovulation.
Because of their earlier formation, approximately 8 to 10 days before the actual ovulation, accessory CLs have a different growth curve from the ovulatory CL (see Fig. 66-2). The accessory CLs are usually more numerous on the ovary of ovulation and reach their maximum diameter approximately 25 to 30 days after the second LH surge, about 10 days earlier than the single CL derived from dominant follicle rupture. After ovulation, the formation of the ovulatory CL also takes about 10 days before luteal tissue may be visualized via ultrasound in the ovary. Although the largest accessory CL measures on average 25 mm (range, 10 to 30 mm), the ovulatory CL is clearly the largest about 40 days postovulation, averaging 33.0 mm (range, 30 to 41 mm).21
Even though LUFs become distinct well before ovulation, progestagen levels only rise to luteal phase concentrations within 1 to 3 days after the second LH peak, but a preovulatory small progestagen rise has sometimes been seen (see Fig. 66-2). This occurs 1 to 3 days prior to the second LH peak and could be derived from the LUF to support ovulation. Regression of accessory CLs occurs approximately 1 week earlier than that of the ovulatory CL, without noticeable changes in progestagen levels. A decline in progestagen concentrations is soon followed by structural luteolysis of the ovulatory CL. Regression of all luteal structures is a slow process and functionally inactive CLs remain visible throughout the next follicular phase and even after the following ovulation, up to 3 weeks into the new luteal phase.21
Concentrations of follicle-stimulating hormone (FSH) rise during the luteal phase and peak at the beginning of the follicular phase, leading to the formation of fresh follicles as soon as progestagen levels are at baseline (see Fig. 66-2). Because inhibin and FSH are negatively correlated, the latter’s concentration declines during the follicular phase and reaches baseline just before ovulation. The inhibin level remains elevated up to 5 weeks into the luteal phase, supporting the theory of luteal origin.19,22
In addition to the very unique formation of the CLs, their secretory capacity is also different from all mammals studied to date. There are only minute quantities of progesterone found, which is the main progestagen in other species. Instead, elephant CLs secrete predominately the reduced 5α-progesterone metabolites 5α-pregnane-3,20-dione (5α-DHP) and 3α-hydroxy-5α-pregnane-20-one (5α-P-3α-OH).17 Correspondent receptors are demonstrated in the endometrium, confirming their biologic significance. The reduced 5α-progesterone metabolites are measurable in excreta and in blood. In African elephants, 5α-P-3α-OH predominates. Also, in the Asian elephants, the 17α-hydroxyprogesterone (17α-OH) metabolite of 5α-pregnanetriol is found in urine and feces, but not in the African species.23
Ultrasound Monitoring During the Estrous Cycle
In addition to the endocrine cycle monitoring, transrectal ultrasonography offers great potential for cycle stage evaluation.11,15 Handheld ultrasound probes of low (4 to 2 MHz) to medium frequency (<7.5 MHz) are useful, but for the visualization of the cranial uterine parts and the ovaries, an extension (handle) for the scanner head might be necessary.15
During the luteal phase, many females develop an anechoic mucous plug within the vagina to protect the uterus from contamination (see Fig. 66-1K). Although the mucus is excreted in a nonconceptive cycle during the follicular phase (usually around the first LH peak, Fig. 66-3A), it is maintained during pregnancy. In the luteal phase, the uterus is relaxed and therefore is usually not totally accessible if the female is standing. The endometrium and myometrium appear less distinct (see Fig. 66-1E). The endometrial layer is thin, with a cross-sectional diameter of only 23.7 ± 0.21 mm as opposed to the 35.4-mm average in the follicular phase (see Fig. 66-1D, F). The ovaries show multiple large CLs (see Fig. 66-1C), of which the accessory CLs typically maintain a fluid-filled central cavity during the first 3 weeks after ovulation (see Fig. 66-1B). Antral follicles never occur during the luteal phase and therefore, in turn, the presence of multiple follicles is a good indicator that a female is in the follicular phase (see Fig. 66-1A).11,21 Furthermore, a higher fluid content in the endometrium caused by low progestagen and increasing estrogen concentrations is noticeable during the follicular phase. Small amounts of free fluid might even accumulate within the uterine lumen 1 to 2 weeks before ovulation. The uterus becomes more toned toward the LH peaks; the horns appear convoluted and are now more easily accessible for the investigator because of retraction and elevation toward the pelvis (see Fig. 66-1G). Because of the more contracted myometrium, the tissues are denser and therefore the uterus appears increasingly bright in the ultrasound image. The endometrium becomes more distinct from the surrounding myometrium and shows a typical echogenicity of interchanging darker and brighter signals shortly before ovulation. After ovulation, the endometrial diameter decreases progressively and reaches the smallest measured thickness of 17 ± 1.5 mm in the early follicular phase of the next cycle.11
Hormone Cycle Induction
Because of the significant differences during the elephant’s estrous cycle (see earlier), endocrine treatments cannot be directly transferred from protocols for domestic animals. However, it is sometimes desirable to control the cycle and induce ovulation, especially for the application of artificial insemination. Information about possible hormonal manipulation is scarce and trials with commercially used drugs have not always produced the expected effects.16
Prostaglandin
Prostaglandin F2α (PGF2α) is commonly used in domestic ruminants and horses to induce luteolysis and initiate a new estrous cycle. In a study on two cycling African elephants, PGF2α was injected once IM at a dose of 80 to 125 mg/elephant on several different days during the luteal phase without any effect on serum progestagen secretion. Only in one female did progestagen levels decline 4 days after the injection, on day 60 postestrus. However, at this time, natural CL regression and termination of the luteal phase occurs (compare summary16).
In another case, PGF2α was administered on three consecutive days to a young pregnant female to induce abortion at month 5 of gestation. Although urinary progesterone metabolite concentrations dropped to baseline during treatment, they returned to normal pregnancy concentrations 10 days after the last injection. A healthy calf was delivered by this dam.24 Therefore, functional luteolysis may be achieved; for structural luteolysis, however, a longer period of PGF2α injection seems to be necessary.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree