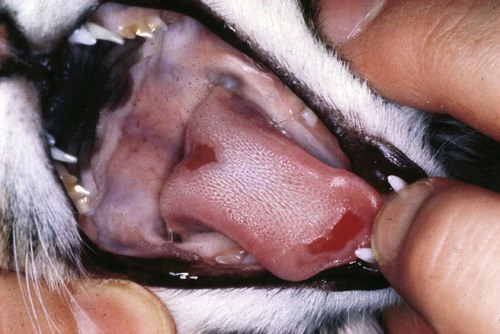
Feline infectious respiratory disease is most commonly seen in cats that are grouped together, as in multicat households, boarding catteries, and breeding establishments. The disease is multifactorial, with several etiologic agents involved and a significant number of other risk factors identified.* The majority of cases of infectious respiratory disease are caused by one of two viruses: feline herpesvirus-1 (FHV-1, or feline rhinotracheitis virus) and feline calicivirus (FCV). FHV-1 generally induces more severe disease than FCV does, but FCV appears to be relatively more common.† This higher prevalence may relate to the antigenic diversity of FCV and the inability of current vaccines to protect equally well against all FCV strains.2,69,90,110,131 Bordetella bronchiseptica, also a primary pathogen of cats, induces respiratory disease in both experimental15,199 and natural6,40,43,67,169 infections. Interestingly, transmission of the organism between dogs and cats may occur, which has implications for disease control in both species (see also Chapter 6).6,8,22,40 Chlamydophila felis (previously Chlamydia psittaci var. felis) is also involved in feline respiratory disease, although it is considered predominantly a conjunctival pathogen (see also Chapter 28). Other agents that have been implicated in the syndrome include mycoplasmas (see Chapter 32) and other bacteria (see Chapter 87), feline reovirus (see Chapter 9), and cowpox virus (see Chapter 17).47,50 FHV-1 is a typical α-herpesvirus containing double-stranded DNA, with a glycoprotein-lipid envelope. As in most herpesviruses, FHV-1 is relatively fragile outside the host and is highly susceptible to the effects of common disinfectants.36,44 It can survive for only up to 18 hours in a damp external environment, less in dry conditions. It is also relatively unstable as an aerosol. As well as infecting domestic cats, FHV-1 has been shown to infect several other members of the Felidae.‡ The virus is also very closely related genetically and antigenically to canine herpesvirus-1 and phocine (seal) herpesvirus-1 (PhV-1), and cross-protection between FHV-1 and PhV-1 has been reported.44,104,104 FHV-1 has little strain variation. Most isolates produce a relatively uniform disease, although some show differences in virulence.44,58 Antigenically, all strains belong to one serotype, and apart from some minor differences, they are relatively homogeneous on restriction enzyme analysis of their DNA.44,57,57 Therefore, no easy method is available to study the role of individual FHV-1 isolates in disease. FCV is a small, unenveloped, single-stranded RNA virus, a member of the Vesivirus genus of the calicivirus family. Both domestic cats and other members of the Felidae family can be infected.20,32,62,83,115 Although dogs have their own genetically distinct calicivirus,105,156 viruses antigenically and genetically related to FCV have also been detected in dogs with diarrhea (see Chapter 8),30,64,103,159 and some epidemiologic evidence suggests a link between the two.7 The role of FCV strains in canine disease and the significance of dogs as a reservoir of feline infection are not known. A large number of different strains of FCV exist, which vary slightly in antigenicity and pathogenicity, although they are all sufficiently cross-reactive to be classified as one serotype.141 Although in general antigenicity is not associated with pathogenicity, some evidence suggests that chronic stomatitis isolates may show minor antigenic differences.23,134 Genetically, FCV strains appear to represent one large diverse group or genogroup, with little evidence of within-group clusters,51,53,122,141 except for two possible genotypes identified in Japan.160 However, considerable variability may be seen between individual isolates, particularly in immunogenic regions of the viral capsid gene.* This genetic diversity is useful epidemiologically in that it allows differentiation between FCV strains.140,142,143,144,178 Most strains of FCV are closely related and induce some degree of cross-protection, but cats can still be sequentially infected with different strains of virus and show varying degrees of clinical illness. Some isolates appear to be more immunogenic and cross-reactive than others. The most commonly used strains in vaccine products have been F9 and 255; however, other strains have also been developed, some for use in bivalent or trivalent FCV vaccines.75,104a,133 There is continued discussion over the degree of cross-protection afforded by such vaccines, particularly the original monovalent FCV vaccines. Some evidence indicates that they are still reasonably cross-reactive against the majority of field isolates, whereas other studies suggest that the percentage of isolates neutralized by such vaccine strains may be decreasing, possibly caused by immune selection pressures from widespread vaccine use.2,11,90,110,131 Direct comparison between studies, however, can be difficult because results may vary according to factors such as the population tested (including perhaps whether sick or healthy cats are sampled75), and the methodology used for the tests. Nevertheless, structured, routine monitoring of vaccine efficacy against current field isolates would be useful. FCV is slightly more resistant than is FHV-1, surviving in the external environment for several days to several weeks on dried surfaces at room temperature, and longer in colder wetter conditions.141 The virus is not as susceptible to the effects of disinfectants as FHV-1,36 but a useful disinfectant for both viruses is household bleach (5.25% sodium hypochlorite) diluted 1 part in 30 in water (1750 ppm) with added detergent.141,196 The susceptibility of FCV to disinfection has been extensively studied because of its close relationship to human noroviruses (see Chapter 93). B. bronchiseptica is an aerobic, gram-negative coccobacillus that is a well-known respiratory pathogen in dogs, swine, and rodents. It also causes occasional opportunistic infections in people: indeed, a case has been reported in a veterinary student40 (see Public Health Considerations, Chapter 6 and Chapters 99 and 100, for further information on this risk.) B. bronchiseptica was thought to play only a secondary role in feline respiratory disease, but it is now established as a primary pathogen in cats. Respiratory disease has been experimentally reproduced in Bordetella-free, specific-pathogen free (SPF) cats after aerosol or nasal challenge,15,74,79,199 and a large number of natural infections associated with clinical illness have been reported.* However, a significant number of factors likely play a role in whether disease occurs under natural circumstances, and various risk factors for B. bronchiseptica infection in cats have been identified in epidemiologic studies.6 For information on the environmental control of this organism, see Management of Outbreaks, Chapter 6. Cp. felis causes acute to chronic conjunctivitis in cats, although respiratory signs may also be seen. The prevalence of Cp. felis in cats with conjunctival or upper respiratory tract disease has been reported in studies using polymerase chain reaction (PCR) to range between 14.3%176 and 59%.10,62a The disease is discussed in more detail in Chapter 28. Reoviruses have been occasionally isolated from the gastrointestinal (GI) or respiratory tract of cats, and conjunctival and respiratory signs have been induced after experimental inoculation. However, no evidence has been found that reoviruses are important as natural respiratory pathogens in cats in the field (see Feline Reovirus Infection Chapter 9). Cowpox virus infection in cats causes primarily skin lesions, but occasionally respiratory or ocular signs may also be seen (see Chapter 17). The reservoir hosts of cowpox virus in Europe are small wild mammals, and cats occasionally become infected by contact through hunting. Other orthopoxviruses that may infect cats exist in other parts of the world, and indeed raccoonpox has been reported in a domestic cat in North America.201 The role of mycoplasmas in feline respiratory disease is not clear (see Chapter 32). Undoubtedly, they can be important as secondary pathogens, but their role as primary agents is more equivocal. Infection is common in both colony cats and household pets, and mycoplasmas have been isolated from both diseased and healthy animals. Increasing evidence indicates that they may be associated with disease in the lower,42,43 and possibly upper respiratory tract, including conjunctivitis.† Other bacteria such as Staphylococcus spp., Streptococcus spp., Pasteurella multocida, and Escherichia coli are thought to play a role as secondary invaders in feline respiratory disease. FHV-1 and FCV are fairly widespread in the general cat population, with a generally higher prevalence in multicat households, in shelters, and also in younger animals.3,7,67,128 The viruses are mainly shed in ocular, nasal, and oral secretions, and spread is largely by direct contact with an infected cat. Acutely infected animals are clearly one of the most important sources of virus, but infection also commonly occurs from clinically recovered carrier cats. In some situations, particularly within a cattery, indirect transmission may also occur. Contaminated secretions may be present on cages, on feeding and cleaning utensils, and on personnel. However, because the viruses are relatively short lived outside the cat, the environment is usually not a long-term source of infection. Some experimental evidence suggests that feces from fleas fed on artificially FCV-infected blood may infect kittens oronasally,109 but this is unlikely to be a significant source of infection. Aerosols are not thought to be of major importance for the spread of FHV-1 and FCV. Cats do not appear to be able to produce an infectious aerosol for these agents during normal respiration, although sneezed macrodroplets may transmit infection over a distance of 1 to 2 m (3 to 6 ft). As with other α-herpesviruses, virtually all recovered cats become latently infected carriers. However, intermittent episodes of detectable viral shedding (reactivation) may occur, particularly after periods of stress (Fig. 14-1). During such episodes, infectious virus is present in oronasal and conjunctival secretions, and cats may infect other cats. Studies using PCR, including real-time PCR, have shown a higher sensitivity for detecting carriers than by use of traditional viral culture techniques. Although the epidemiologic significance of those cats that are positive for FHV-1 by PCR but negative on viral isolation is not clear, they are likely to be less infectious.9,44,177,193 Viral reactivation may occur spontaneously but is most likely after stress, for example, going into a boarding or rescue cattery, to a cat show, or to stud.44 Once reactivation occurs, depending on factors such as hygiene precautions, infection can spread rapidly, particularly in shelter environments.3,84,84 Glucocorticoid treatment can also induce shedding in FHV-1 carriers, but using this drug to detect carriers is inadvisable because severe disease may occasionally result. Some carrier cats appear to shed virus more frequently than do others and, therefore, are of greater epidemiologic importance. Shedding does not occur immediately after the stress; a lag period of approximately 1 week occurs, followed by a shedding episode of from 1 to 2 weeks.44 Thus carrier cats are most likely to be infectious for up to 3 weeks after a stress factor. In some cases, carriers show recrudescence of mild clinical signs while they are shedding, which can be a useful indicator that they are likely to be infectious. The stress of parturition and lactation may also precipitate viral shedding in latently infected queens, but whether or not the kittens develop disease depends on their levels of maternally derived antibody (MDA). On some occasions, kittens with low levels of MDA may become subclinically infected and become latent carriers without showing clinical signs.44 Such a mechanism is obviously ideal for the virus because it can spread to the next generation without harming its host. As with some other herpesviruses, FHV-1 remains latent in carriers in the trigeminal ganglia, although virus has also been detected by PCR in other tissues.124,153,153 The latent carrier state is almost certainly lifelong, but a refractory phase of several months occurs after a period of viral shedding when animals are less likely to experience another reactivation episode.44 The carrier state appears to be widespread in the cat population, with approximately 10% of household pets and 25% to 75% of shelter or colony cats being positive for FCV (Fig. 14-2).16,67,131,141,203 The virus persists in carriers in tonsillar and other oropharyngeal tissues. Although the precise mechanism of persistence is unclear, it is likely to include immune selection pressure driving antigenic variation in the viral capsid protein, which then allows the virus to evade the host immune response.18,86,86 However other host, viral, and environmental factors also undoubtedly play a role. In situations such as boarding and rescue facilities, many different FCV strains tend to be present, probably reflecting the relatively rapid turnover of carrier cats bringing in their own individual strains from different sources.17,143 In contrast, in colonies of cats where FCV is endemic, in general only one or two distinct strains circulate.16,18 Longitudinal studies of these endemically infected colonies have shown that although many cats appear to be long-term carriers, only a small minority (approximately 10%) are truly persistently infected, the remainder undergoing cycles of reinfection from within the colony.18 Those with true persistent infection continuously evolve their own viral strain over time, and unlike FHV-1 carriers, there appears to be no latent phase for such FCV carriers.16 The remainder of long-term carriers appear to undergo cycles of reinfection, either with a variant of the same strain, or in some cases, with a different strain circulating within the colony. Interestingly, some cats within these endemic situations appeared to be resistant to infection, suggesting either age-related or genetically determined resistance mechanisms.16 These findings help explain first, the lower carriage rate of approximately 10% found in household pets, where the opportunity for reinfection is less likely to occur; and second, the inability in some previous experimental studies to induce a carrier state in small groups of cats. Serosurveys have shown that B. bronchiseptica is widespread in the cat population. Seroprevalence of between 24% and 79% and isolation rates of up to 47% have been reported, depending on the type and clinical status of the population tested.* In a large-scale epidemiologic survey of cats in the United Kingdom with and without respiratory disease, 11% of 740 cats were found to be shedding B. bronchiseptica from the oropharynx, with a higher positive number in rescue catteries and in households with larger numbers of cats.6 An association between B. bronchiseptica infection and respiratory disease was found in the rescue cattery population, which fits with previous empirical observations that the organism is more likely to cause disease in stressful, crowded situations. Also apparent is that transmission of this bacterium may occur between dogs and cats, which clearly has implications for control, especially where dogs and cats are housed on the same premises. Epidemiologic studies have shown that contact with dogs with recent respiratory disease was found to be a risk factor for B. bronchiseptica infection in cats.6 In addition, typing of isolates from dogs and cats using pulsed field gel electrophoresis has shown that isolates from both species on the same premises are likely to be similar.8,40 In another report, 2 cats developed respiratory disease after contact with 2 dogs with kennel cough, and B. bronchiseptica isolated from all 4 animals were found to be the same using pulsed field gel electrophoresis.22 Interestingly, Foley and colleagues40 noted that the majority of isolates circulating in dogs and cats in two shelters in the United States appeared to be similar to a canine and feline vaccine strain. Epidemiologic evidence suggests that a carrier state may exist in cats with Bordetella infection, with 7% to 9% of clinically healthy cats shedding the organism.6,112 Experimental work has shown that B. bronchiseptica is shed in both oropharyngeal and nasal secretions, in some cases for at least 19 weeks after infection.15 In the same study, B. bronchiseptica was detected in two seropositive queens after parturition despite being negative beforehand, suggesting the stress of parturition may have initiated detectable shedding. The natural routes of infection for FHV-1 are nasal, oral, and conjunctival, and viral replication takes place predominantly in the mucosae of the nasal septum, turbinates, nasopharynx, and tonsils. Viral shedding can be detected in oropharyngeal and nasal swabs as early as 24 hours after infection and generally persists for 1 to 3 weeks. Viremia is rare because virus replication is normally restricted to areas of lower body temperature, such as the respiratory tract. However, viremia has been reported, and generalized disease may be seen, particularly in debilitated animals or in neonatal kittens.44 Similar to FHV-1, the natural routes of infection for FCV are nasal, oral, and conjunctival. Viral replication mainly occurs in the oral and respiratory tissues, although some differences between strains can be found. Some strains have a predilection for the lung, and others have been found in the macrophages within the synovial membrane of joints.21,183 Virus may also be found in visceral tissues and feces and occasionally in the urine. Oral ulcers are the most prominent pathologic feature of FCV infection.141 These ulcers begin as vesicles, which subsequently rupture, with necrosis of the overlying epithelium and infiltration of neutrophils at the periphery and the base. Healing takes place over 2 to 3 weeks. Lesions seen in FCV-infected joints consist of an acute synovitis with thickening of the synovial membrane and an increased amount of synovial fluid within the joint. In cases of FCV-associated virulent systemic disease (see later discussion), virus gains access to cellular compartments not normally associated with FCV.129 Lesions are widespread and include subcutaneous edema, ulceration of the mouth, and variable levels of ulceration of the skin particularly on the pinnae and pawpads and nares.19,76,126,154,162 Other lesions are more variable and include bronchointerstitial pneumonia and necrosis in the liver, spleen, and pancreas. Viral antigens have been detected in the skin, nasal mucosa, lung, pancreas, and endothelial cells of the dermis associated with necrosis.130 In another study, viral antigen was found in livers of jaundiced cats.19 In most species, the primary route of infection with B. bronchiseptica appears to be via the oronasal cavity, where the organism colonizes mucosal surfaces.169 The bacterium uses several virulence factors to adhere to the cilia of the respiratory epithelium. Once attached, ciliostasis and destruction of the cilia occur, resulting in the failure of the mucociliary clearance mechanism and facilitating further colonization and persistence of bacteria. The release of toxins from B. bronchiseptica after colonization is responsible for local and systemic inflammatory damage. In dogs, the organism appears to target mainly the mucosa of the trachea and bronchi, leading to tracheobronchitis (see Chapter 6). Although the precise pathogenesis of the disease in cats is unclear, upper respiratory tract involvement appears to be more common. However, clearly in some instances, the lower respiratory tract may also be targeted, given that bronchopneumonia and coughing may occur. Although B. bronchiseptica may appear to be a primary pathogen in cats, undoubtedly other factors such as combined infections with the respiratory viruses and stress factors such as weaning, overcrowding, and poor hygiene and ventilation all play roles. Such factors may account for the severe cases of bronchopneumonia that have been reported in the field. Whatever the respiratory pathogen, the observed clinical signs will depend on a large number of factors, such as the infecting dose and strain of the agent, the general health and husbandry conditions of the cat, the nature of its microbial flora, and any preexisting immunity (Table 14-1). Concurrent infection with immunosuppressive viruses such as feline immunodeficiency virus and feline leukemia virus may lead to more severe disease.24,150,150 TABLE 14-1 Essential Features of Clinical Respiratory Diseasea Related to the Pathogen Involved +++, Marked; ++, moderate; +, mild; (+), less common; +/–, subclinical; –, absent; Bb, Bordetella bronchiseptica; ChF, Chlamydophila felis; FCV, feline calicivirus; FHV-1, feline herpesvirus. aFor FCV-associated virulent systemic disease signs, see text. dSlight wetness may be seen around the mouth if ulcers present. Adapted from Ref. 50. Used with permission. In susceptible animals, FHV-1 infection generally causes a severe upper respiratory disease. The incubation period is usually 2 to 6 days but may be longer with lower levels of challenge virus.44 Early signs include depression, marked sneezing, inappetence, and pyrexia, followed rapidly by serous ocular and nasal discharges (Fig. 14-3). These initial clinical signs may be accompanied by excessive salivation with drooling. Conjunctivitis, sometimes with severe hyperemia and chemosis, typically develops, and copious oculonasal discharges occur. These discharges gradually become mucopurulent, and crusting of the external nares and eyelids can occur (Fig. 14-4). In severe cases, dyspnea and coughing may also develop. Oral ulceration can occur with FHV-1 infection, but it is rare. Occasionally, generalized infections and primary viral pneumonia may occur, particularly in young or debilitated animals, and very rarely neurologic signs have been described.44 In very young kittens or immunosuppressed cats, the mortality rate may be high, but on the whole, mortality with FHV-1 is low. Involvement of FHV-1 in conjunctivitis and, in some cases, ulcerative keratitis (Fig. 14-5) has long been known,44 but improved viral detection using PCR has led to increased recognition of this role in the acute disease, and also in more chronic ocular lesions such as stromal keratitis.* The role of FHV-1 in other ocular conditions such as corneal sequestrae, eosinophilic keratitis, uveitis, and keratoconjunctivitis sicca is, however, less clear and needs further evaluation.97,118,172,173,191 Skin ulcers and an ulcerative facial and nasal dermatitis and stomatitis syndrome characterized by eosinophilic infiltration, which is occasionally persistent, has been described in cats, with a similar syndrome also being reported in cheetahs.60,61,70,115 However, in one study involving domestic cats,91 FHV infection was found in only 2 of 30 cats with eosinophilic granuloma complex. Although abortion is a feature of some other α-herpesvirus infections, experimental studies have suggested that, in FHV-1, abortion is most likely due to the severe systemic effects of the illness, rather than a direct effect of the virus itself.44 Indeed, in an investigation of a natural outbreak of FHV-1 in SPF cats, no cases of abortion were seen, even in severely affected pregnant queens.68 The clinical signs of FHV-1 infection generally resolve within 10 to 20 days. However, in some cats, the acute damage may have been severe enough to lead to permanent damage to the mucosae and turbinates, leaving individual cats prone to chronic bacterial rhinitis, turbinate osteomyelitis, sinusitis, and conjunctivitis. Because of the propensity of FHV-1 to replicate in the turbinates in the acute phase of the disease, and in the early stages of reactivation, a continuing role of the virus itself has been considered in chronic rhinitis. However, a study has found no difference in detection rate between cases and controls,81 and it is likely that this syndrome is more related to the damage caused in the acute disease and subsequent chronic bacterial infection. Short-nosed purebred cats such as Persians or Himalayans appear to have an increased tendency to develop chronic upper respiratory complications, although the reasons for this are not yet clear. Strains of FCV can differ in tropism and virulence; therefore, a wide range of clinical signs may be seen. Most strains induce a fairly characteristic, mild syndrome characterized by pyrexia, oral ulceration, and mild respiratory and conjunctival signs. Some strains of FCV, however, are nonpathogenic; others are more virulent and may induce more severe systemic disease. Several outbreaks of a severe acute systemic febrile disease with high mortality have been described, particularly in the United States and in Europe, caused by particularly virulent strains of FCV (FCV-associated virulent systemic disease [FCV-VSD]).* In a typical case of FCV infection, early signs include depression and pyrexia, although cats typically stay brighter than with FHV-1 infection. Oral ulceration is the most characteristic feature of FCV infection and may be the only clinical sign present, and it is possible that many cases go unreported (Fig. 14-6). Ulceration is usually on the tongue but can occur elsewhere in the mouth, on the lips, and on the nose. Skin ulceration on other parts of the body occurs rarely. Sneezing, conjunctivitis, and ocular and nasal discharges typically occur but are generally much less prominent compared with FHV-1 infection. Cats with oral ulcers may show hypersalivation with moisture on the fur around the mouth, but generally, no drooling of saliva occurs. Some strains can cause pneumonia with associated dyspnea. Other FCV isolates produce a febrile disease with lameness and pyrexia.181 The lameness may or may not be associated with oral and respiratory signs, and it is likely that considerable overlap exists between the conditions.181 Affected cats are often dull and anorexic. In most cases, full recovery occurs within 24 to 48 hours, and so far, no evidence can be found of any long-term effects on the joints. Acute lameness has also been observed after vaccination, and in some of these cases, viruses originating from live vaccines may be involved.142 Most of the outbreaks of FCV-VSD are nosocomial infections, and each outbreak appears to have been caused by a different FCV strain.† Although highly contagious within a group of cats, all outbreaks appear to have been contained, and the disease has not spread into the general population. Possible reasons for the appearance of such strains and for their subsequent disappearance are not understood but have been discussed elsewhere.141,154 High mortality among affected cats and disease containment may be two important factors limiting spread of these outbreaks, though other factors may play a role.141 Cats with FCV-VSD variably show facial and paw edema, pyrexia, classic signs of oral ulceration and upper respiratory infection, icterus, and hemorrhages from the nose and in the feces, and mortality is high. Necropsy findings are again somewhat variable in individual cases but include pneumonia, hepatomegaly, pancreatitis, and pericarditis.19,76,126,154,162 Many of the affected cats are fully vaccinated, suggesting that, as can occur with more typical strains of FCV, vaccines may not completely protect against these virulent systemic disease-producing isolates. Although the outbreaks have been relatively well controlled with strict quarantine and isolation, given that FCV is an inherently variable pathogen, clinicians must remain vigilant in the event of further outbreaks of this disease. The chronic oral disease feline chronic gingivostomatitis complex has also been associated with FCV infection. The prevalence of this condition has been estimated at 0.7% of consultations in first opinion practice in the United Kingdom65 and can be a particular problem because of the difficulty in treating this condition. In a number of studies, more than 80% of cats with chronic oral disease have been found to shed FCV compared with approximately 20% of controls,141 but these figures may vary depending on the criteria used to select clinical cases.180 FCV infection has been associated with acute faucitis,150 and in one colony of cats, chronic stomatitis developed after the accidental introduction of FCV.192 However, other agents, in particular feline immunodeficiency virus, and various host factors appear to be involved in the condition, and the pathogenesis is not yet fully understood (see Feline Lymphocytic Plasmacytic Ulceroproliferative Gingivostomatitis (Faucitis) Chapter 88). In naturally occurring disease, a wide range of clinical signs have been reported in cats infected with B. bronchiseptica, varying from upper respiratory tract signs of sneezing, oculonasal discharge, and coughing to severe dyspnea, cyanosis, and death caused by bronchopneumonia.6,22,169,184,194 In general, coughing appears to be less marked in cats than it is in dogs infected with B. bronchiseptica. Young kittens appear to be most susceptible to disease, particularly bronchopneumonia. In experimental studies using SPF cats, the main clinical signs of B. bronchiseptica infection were pyrexia, lethargy, coughing, sneezing, oculonasal discharge, and submandibular lymphadenopathy, which resolved after approximately 10 days.15,73,74,79,199 Diagnosis may be attempted based on clinical signs alone. For example, predominantly oral ulceration might indicate FCV, whereas marked sneezing with more severe respiratory and conjunctival signs might suggest FHV-1 (see Table 14-1). With Chlamydophila infection, the main clinical sign is a marked persistent conjunctivitis (see Chapter 28). Confirmatory diagnosis of FHV-1 and FCV is generally made using oropharyngeal swabs, though conjunctival or other samples may sometimes be used. Traditionally, viral isolation in feline cell cultures has been used to detect FHV-1 and FCV. Less commonly, immunofluorescence has also been used, particularly for the detection of FHV-1 in conjunctival or corneal smears.182 PCR is commonly used for detection of FHV-1 because it is generally more sensitive than viral isolation, especially in the more chronic stages of infection.44,177,177 However, PCR techniques from different laboratories vary considerably in sensitivity,95 and a positive result should be interpreted within the clinical context (see later discussion). For FCV, several reverse transcriptase PCRs have now been described, using various approaches including targeting different regions of the genome.* These assays are still not widely used for diagnosis, because of concerns that the strain variability of FCV may lead to false-negative results. Despite this, PCR and sequencing, particularly of the hypervariable region of the capsid gene, are useful for distinguishing between FCV isolates and have been extensively used to better understand the epidemiology of the disease.140,142,143,144 Serology is generally not helpful in the diagnosis of FHV-1 or FCV infection because of widespread antibody from vaccination. For diagnosis of B. bronchiseptica infection either bacterial culture or PCR can be used.67,169 For culture, oropharyngeal or nasal swabs should be taken and placed into charcoal Amies transport medium (Beckton Dickinson, Cockeysville, MD) before plating at the laboratory on appropriate selective medium that prevents overgrowth by other respiratory flora.169,189 Transtracheal wash specimens or bronchoalveolar lavage can also be used for the isolation of B. bronchiseptica from clinically affected cats. Serology is not widely available, and many healthy cats are seropositive in any case. Diagnosis of Cp. felis infection is described in Chapter 28. Cp. felis infection should be considered when conjunctivitis is the predominant clinical sign. When an organism is detected in an animal with respiratory disease, it is reasonable to assume that the agent is involved in the disease process. However, especially for FCV and Bordetella, a relatively large number of clinically healthy cats will also be shedding the organisms; thus, interpretation of a positive result may be problematic unless taken in conjunction with other factors, such as the characteristic clinical signs. Similarly for FHV-1, latently infected cats may also shed reactivated virus, and thus a positive result may also be unrelated to the observed clinical signs. In an epidemiologic context, however, the positive result indicates the cat has been infected with FHV-1 and thus has the potential to infect other cats. For FHV-1, quantitative real-time PCR can provide additional information with respect to viral load and stage of infection, especially if consecutive samples are taken.190 In countries where intranasal vaccines are available, a positive result may be indicative of FHV-1 or FCV vaccine virus rather than wild-type viruses.
Feline Respiratory Disease
Etiology
Feline Herpesvirus-1
Feline Calicivirus
Bordetella bronchiseptica
Other Organisms
Epidemiology
Feline Herpesvirus-1 and Feline Calicivirus Infections
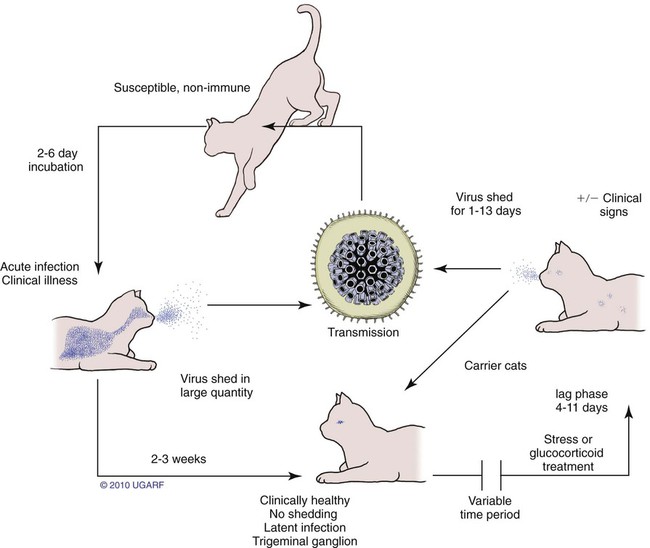
Carrier State for Feline Herpesvirus-1
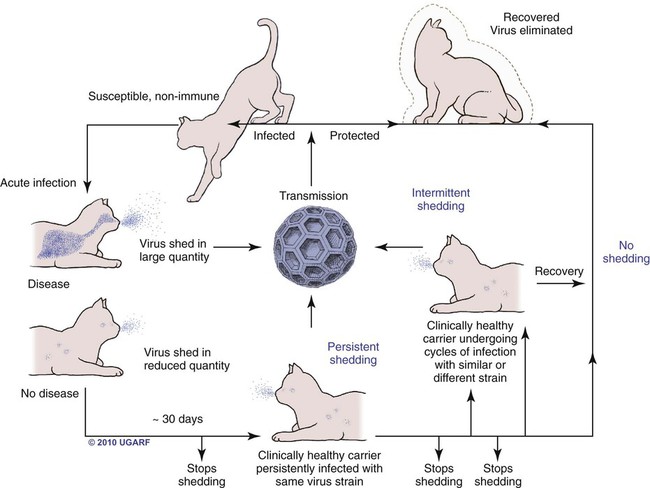
Carrier State for Feline Calicivirus
Bordetella bronchiseptica Infection
Pathogenesis
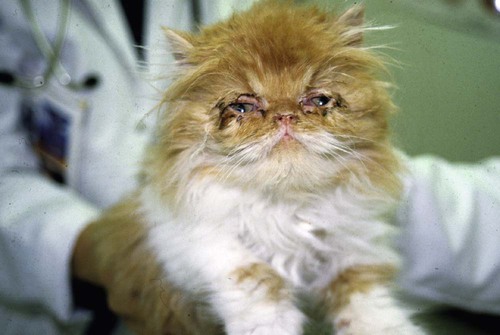
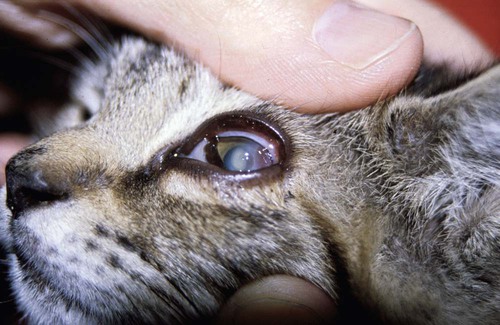
Feline Herpesvirus-1
Feline Calicivirus
Bordetella bronchiseptica
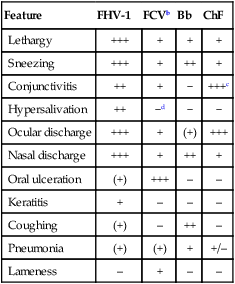
Clinical Findings
Feature
FHV-1
FCVb
Bb
ChF
Lethargy
+++
+
+
+
Sneezing
+++
+
++
+
Conjunctivitis
++
+
−
+++c
Hypersalivation
++
−d
−
−
Ocular discharge
+++
+
(+)
+++
Nasal discharge
+++
+
++
+
Oral ulceration
(+)
+++
–
–
Keratitis
+
–
–
–
Coughing
(+)
–
++
–
Pneumonia
(+)
(+)
+
+/–
Lameness
–
+
–
–
Feline Herpesvirus-1 Infection
Feline Calicivirus Infection
Bordetella bronchiseptica Infection
Diagnosis
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree