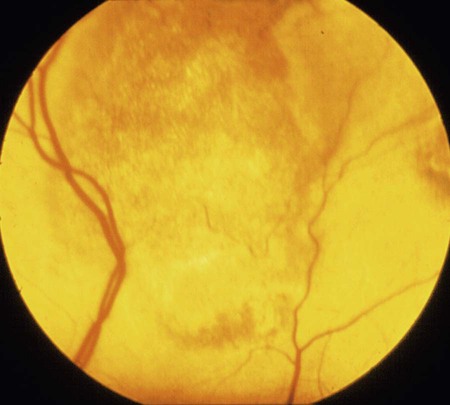
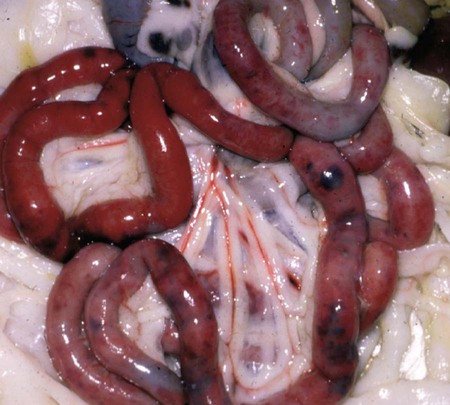
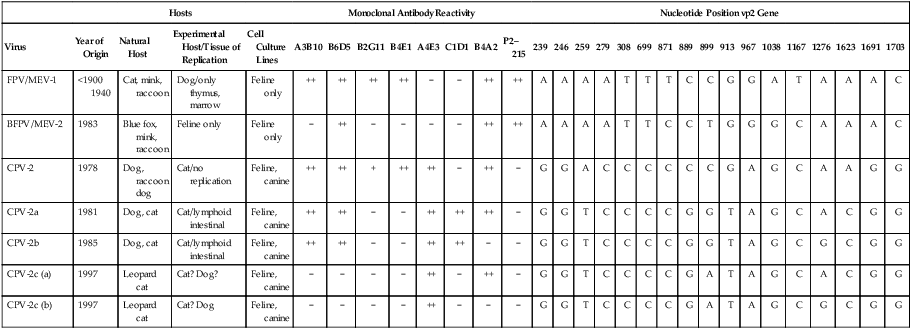
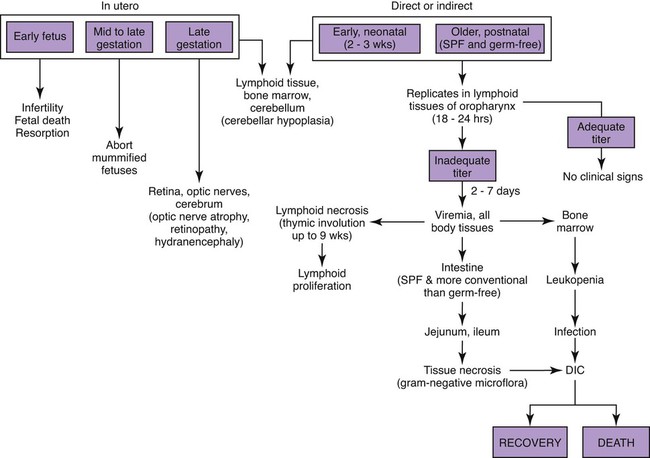
Feline panleukopenia is caused by a small, serologically homogeneous parvovirus (feline parvovirus [feline panleukopenia virus, FPV]), with single-stranded DNA. Small differences in the genome have been detected among viral isolates from the same and different hosts7,21,21; however, these appear to be clinically insignificant random mutations. Genetically, structurally, and antigenically, it is closely related to blue fox parvovirus, mink enteritis virus (MEV), and canine parvovirus (CPV) (Web Table 9-1).73 In addition, CPV strains 2a, 2b, and 2c have been isolated from healthy cats75 and from those with signs of feline panleukopenia (see later discussion, Canine Parvoviral Infection in Cats, and Chapter 8).6,7,78,126 In addition to genetic variations reported among FPV isolates, some evidence of genetic recombination between CPV2 and FPV isolates has been observed.77,91 In reported surveys for parvoviral enteritis in cats, the prevalence of FPV infection is over 95% of cases compared to those involving CPV-2a, CPV-2b, or CPV-2c.21,34 In contrast to CPV strains infecting cats, FPV has limited replication (lymphoid tissues) in dogs after experimental inoculation; it does not infect the gut epithelium and, therefore, is not shed.128 A unique FPV strain, closely related to those infecting cats, was isolated from the diarrheic feces of a monkey among Macaca spp. monkeys suffering from a severe hemorrhagic diarrhea outbreak in China.134 It produced characteristic feline panleukopenia in experimentally infected cats. Further studies will be needed to determine if the isolate is pathogenic in monkeys. WEB TABLE 9-1 Comparison of Parvoviral Isolates A, Adenine; BFPV, blue fox parvovirus; C, cytosine; CPV, canine parvovirus; FPV, feline panleukopenia virus; G, guanidine; MAB, monoclonal antibody; MEV, mink enteritis virus-1; T, thymidine; ++, strong; +, weak; −, negative; ?, unknown. FPV is very stable, able to survive for 1 year at room temperature in organic material on solid fomites. Outdoors, parvoviruses in fecal organic material can survive for 5 to 10 months or more; however, heat and drying in the summer months or removal of organic debris accelerates its inactivation.129 FPV resists heating to 56° C for 30 minutes and remains viable for longer periods at lower temperatures. The virus survives disinfection with 70% alcohol and various dilutions of organic iodines, phenolics, and quaternary ammonium compounds. Parvoviruses such as FPV are inactivated by bleach (5.25% sodium hypochlorite) dilutions (see Chapter 93), 4% formaldehyde, peracetic acid, sodium hydroxide (0.1 M at pH 12.8 or higher), and 1% glutaraldehyde in 10 minutes at room temperature. For heat disinfection of parvoviruses, temperatures of at least 90° C are required for 10 minutes.10 FPV can cause disease in all members of the family Felidae, and numerous reports of infection or exposure in nondomestic cats exist.26,69,102,132 Some Viverridae, Procyonidae, and Mustelidae, including the binturong, raccoon, coatimundi, ring-tailed cat, and mink, are also susceptible (see Table 100-10). The virus is ubiquitous because of its contagious nature and capacity for persistence in the environment. Virtually all susceptible cats are exposed and infected within the first year of life. Unvaccinated kittens that acquire maternally derived antibody (MDA) through colostrum are usually protected up to 3 months of age (although longer duration of MDA to 20 weeks sufficient to interfere with vaccination has been reported).2 Most infections are subclinical, inasmuch as 75% of unvaccinated, clinically healthy cats have demonstrable antibody titers by 1 year of age. Seasonal variations in the incidence of panleukopenia and disease outbreaks presumably parallel increases in the number of susceptible newborn kittens. Although panleukopenia is regarded as a condition of unvaccinated random-source cats, infection has been reported in kittens born into pedigree breeding cats from well-vaccinated queens.1a,2,15 A perception exists among veterinary practitioners that the prevalence of FPV infection in cats has diminished over the past 2 decades. Reasons for this decrease may be the more widespread vaccination of cats and the use of modified live virus (MLV) vaccines, which may cause viral shedding, thereby immunizing exposed cats. Second, the newly emerging CPV-2 strains infecting cats may offer some cross-protection against FPV infection. FPV may be more adapted to its host; however, other prenatal or neonatally acquired forms of disease such as neurologic or cardiac manifestations are occurring (see later discussion under Pathogenesis). Because of its short shedding period, but long environmental survival, FPV is most commonly transmitted by indirect contact of susceptible animals with contaminated premises. It is shed from all body secretions during active stages of disease but is most consistently recovered from the intestine and feces. Similar to CPV infection in dogs during the acute intestinal phase of illness, cats infected with virulent virus can shed up to 109 viral particles per gram of feces. Viral shedding usually lasts 1 to 2 days, but cats can shed virus in their urine and feces for a maximum of 6 weeks after recovery.18 FPV is maintained in the population by its environmental persistence rather than by prolonged viral shedding. In utero transmission occurs. The virus has been isolated for a maximum of 1 year from the kidneys of neonatally infected kittens, but urinary shedding does not occur. Owners who lose a kitten to feline panleukopenia should not introduce a new kitten into the household without having it vaccinated prior to entry. As a parvovirus requiring cellular DNA polymerase, FPV requires rapidly multiplying cells, in the S-phase of division, for successful infection to occur. The distribution of lesions within a prospective feline host occurs in tissues with the greatest rate of mitotic activity (Web Fig. 9-1). Lymphoid tissue, bone marrow, and intestinal mucosal crypts (intestinal glands) are most commonly invaded in adult animals. Late prenatal and early neonatal infections in cats result in some lymphoid and bone marrow lesions, but the central nervous system (CNS), including the cerebrum, the cerebellum, the retina, and optic nerves, can be affected. During intestinal infection, the virus selectively damages replicating cells deep in the crypts of the intestinal mucosa. Differentiated absorptive cells on the surface of the villi are nondividing and are not affected. Shortening of the intestinal villi results from damage to the crypt cells, which normally migrate up the villi, replacing absorptive cells. Damage to the intestinal villi results in diarrhea caused by malabsorption and increased permeability (see Fig. 88-6, C). Concurrent infections with copathogens can increase the severity of FPV infections in cats, similar to the situation in dogs with CPV-2 infection. Intestinal cell replication increases during insults to the bowel mucosa, which can increase the virulence of parvoviruses that infect rapidly dividing cells. Dual infection with Clostridium piliforme, the causative agent of Tyzzer’s disease, was found in kittens (see also Chapter 37).59 Co-infections with salmonellae and FPV have also been described in purebred catteries, with severe mortality.33 FPV may be an immunosuppressive agent in these enteric bacterial co-infections, given that it causes both leukopenia and bowel injury, which allows bacterial proliferation. In contrast, co-infection with a nonenteric pathogen such as cowpox virus caused no increase in severity of either viral infection.107 However, a combined infection of feline calicivirus (FCV) and FPV was associated with a high severity of illness.13 Early in utero infection can produce a spectrum of reproductive disorders in the pregnant queen, including early fetal death and resorption with infertility, abortions, or the birth of mummified fetuses. Closer to the end of gestation, infections will result in birth of live kittens with varying degrees of damage to the late-developing neural tissues. FPV produces variable effects on animals from the same litter. Some kittens are apparently unaffected owing to either innate resistance or the acquisition of MDA, but they may harbor virus subclinically for up to 8 to 9 weeks in some cases.18 The CNS, optic nerve, and retina are susceptible to injury by virulent or vaccine strains of FPV during prenatal or early neonatal development; of CNS lesions, cerebellar damage has been most commonly reported. This predilection for cerebellar disease can be explained by the fact that in cats the cerebellum develops during late gestation and early neonatal periods. FPV interferes with cerebellar cortical development, resulting in reduced and distorted cell layers. The cerebellum can be affected by infections occurring as late as 9 days of age. Parvovirus can be detected in the CNS of affected cats by immunochemical staining.3 Polymerase chain reaction (PCR) has confirmed that FPV DNA is found in the cerebellums of cats with cerebellar hypoplasia.101,109 Other CNS lesions can be produced by earlier prenatal infections. Lesions of the spinal cord and cerebrum, including hydrocephalus, hydranencephaly, and optic nerve and retinal abnormalities, can occur (see Clinical Findings later in this chapter).14,38,43,98,115 Purkinje’s cells of the cerebellum are particularly susceptible to FPV infection, presumably because they express transferrin receptor that is used by parvovirus for cell entry. Although they should not allow viral replication, being postmitotic cells, transcription of viral proteins occurs as viral antigen can be detected in these cells up to 3 weeks after experimental infection of newborn kittens.17 Purkinje’s cell degeneration in the cerebellum was described in one adult feral cat with systemic FPV infection.31 Unexpectedly, parvoviruses also appear to be capable of replicating in neurons, which are considered to be terminally differentiated cells. Cats that had died of various diseases including panleukopenia had parvovirus detected histochemically in their brains.130 These cats did not have the clinical signs of cerebellar hypoplasia, nor were they of that susceptible age group. Viral nucleic acid was found by in situ hybridization to be in brain nuclei, especially in the diencephalic areas. Some of the virus appeared to be CPV-2 of the old antigenic type. The clinical significance of this neuronal infection is unknown. Myocarditis in humans and animals can be induced by a large number of viruses. Myocarditis was one of the early recognized features of CPV-2 infections in dogs, and it continues to be a feature of CPV-1 infection in dogs (see Chapter 8). With CPV-2, a lack of maternal immunity, during the early epidemic period of the late 1970s and before the advent of available vaccines, resulted in increased susceptibility of the fetus to infection. FPV likely infects kittens born to queens that were exposed to the virus during pregnancy. Hearts from cats dying of idiopathic hypertrophic, dilated, and restrictive forms of cardiomyopathy were examined by PCR for genomic evidence of FPV, FCV, feline herpesvirus-1, and feline coronavirus.71 Only FPV was identified in a significant number of the hearts. These data suggest that FPV is important in the pathogenesis of this disease in cats. The frequency with which cats show evidence of clinical disease with FPV is much less than the number of cats infected with the virus. This fact is supported by the high prevalence of FPV antibodies in the cat population. Subclinical cases, more common in older susceptible cats, remain unrecognized. Severe clinical illness is the rule in young unvaccinated kittens; the highest morbidity and mortality occurs between 3 and 5 months of age. Sudden neonatal or adolescent death (fading kittens) has been observed in kittens of 4 weeks to 12 months of age from households of vaccinated pedigree cats.2 In one study in the United Kingdom, FPV was the cause of 25% of kitten mortality.15 The acute form is most common, with fever (40° C to 41.6° C [104° F to 107° F]), depression, and anorexia occurring within 3 to 4 days before presentation. Vomiting, which develops during the illness in most cats, is frequently bile-tinged and occurs unrelated to eating. Extreme dehydration, sometimes exhibited by the cat crouching with its head over the water dish, may occur as a nonspecific feature of this disease (Fig. 9-1). Diarrhea occurs with less frequency. When it is present, the diarrhea usually occurs somewhat later in the course of illness. Queens infected or vaccinated during pregnancy may show infertility or abortion of dead or mummified fetuses, but clinical signs are never exhibited in the aborting female. Some kittens in a litter may be born with ataxia, incoordination, tremors, and normal mental status typical of cerebellar disease (Fig. 9-2). They walk with a broad-based stance with hypermetric movements, and they frequently show intention tremors of the head. Tremors and incoordination are absent when kittens are at rest. Not all kittens in a litter are affected or have the same degree of neurologic deficits.115 Signs of forebrain damage from hydranencephaly include seizures, behavioral changes, and relatively normal gait despite postural reaction deficits. Affected kittens with minimal cerebellar dysfunction can compensate to a degree with time and may make suitable pets with subtle residual deficits. Retinal lesions may be visible on fundic examination of kittens affected with neurologic signs or as an incidental finding in clinically healthy cats.38,98 These areas of retinal degeneration appear as discrete, gray foci with darkened margins, and retinal folding or streaking may be seen (Fig. 9-3). Feline salmonellosis with overwhelming septicemia may mimic feline panleukopenia with the presence of leukopenia and acute gastrointestinal illness. Fecal culture can be helpful under these circumstances (see Chapter 37). A transient decrease in absolute reticulocyte count and a mild (5% to 10%) decrease in hematocrit have been found during the viremic period in experimentally infected kittens. Because of the sudden onset of the disease and relatively long life span of erythrocytes, marked anemia is also less common in panleukopenia unless intestinal blood loss is severe. A persistent, nonregenerative anemia and leukopenia are more suggestive of feline leukemia virus (FeLV) infection (see Diagnosis, Chapter 11). Magnetic resonance imaging or computed tomographic scanning can be used to visualize cerebral or cerebellar cortical defects in cats with neurologic signs resulting from in utero infections.115 Quantitative procedures are available for the properly equipped diagnostic and research laboratory, although they are rarely indicated for clinical practice. Single sample antibody titers do not distinguish between active infection or past exposure to virulent or vaccine virus. Defined levels of titers can be established that measure protection against natural infection. Serum VN is the most common and reference method employed. Twofold serial dilutions of antisera are performed against precalculated amounts of FPV. Virus and sera are incubated before inoculation of the cell culture. Cultures can be examined for specific cytopathic changes and inclusion bodies produced by the virus. The first sample is taken as soon as possible during the illness, and the second is taken 2 weeks later. A fourfold rise in VN titer is indicative of acute infection. Complement fixation titers also can be performed. Hemagglutination inhibition (HI) and hemagglutination tests can be performed using some strains of FPV, which, as with CPV, will variably agglutinate porcine erythrocytes at 0° C but at pH 6.4 rather than 7.2. The variation is usually related to individual variation among pig erythrocytes in the test. A fourfold rising titer is considered indicative of active infection. VN and HI titers have been used as the reference standards for protection against infection.67,112 Minimum titers (above 10) that correlate with resistance to challenge infection have been determined in these studies. The highest titers are consistent with natural exposure to virus, as few cats mount these titer responses after vaccination. In one study,67 the enzyme-linked immunosorbent assay (ELISA) method was too sensitive in that positive titer results were detected in a low number of unvaccinated cats that were not protected following challenge infection. Parvoviral antigen can be detected in feces using immunologic methods. ELISA-based tests, commercially marketed for detecting CPV antigen in feces or intestinal contents, are a sensitive and practical indicator of FPV infection in kittens.1,1a,,2,30,87 Commercially available kits are licensed for detection of CPV available for this purpose (see Web Appendix 6). Results using fecal ELISA kits for CPV are usually weaker in intensity than following natural virulent virus infection. Results may remain positive for up to 2 weeks after administration of MLV vaccines.87,97 Accuracy in detecting virulent virus may vary between these commercial assays2 so their preliminary evaluation is advisable. Immunoassays have also been performed on tissue specimens taken at necropsy. These are homogenized, and the supernatant has been applied directly on the test strip.2 However, it must be borne in mind that FPV may be detectable only in the feces by ELISA kits for 24 to 48 hours postinoculation, and that by the time clinical signs occur, the virus may no longer be detectable. Feline cells are required to support viral replication in cell cultures, and frequent mitosis is needed to ensure a continuing infection, although FPV has been shown to replicate in cells in which DNA synthesis has been blocked. Cytopathic effects, required to substantiate the presence of the virus, are more easily demonstrated in young, rapidly multiplying cells. Plaque-detection methods are possible when certain cell types and cell synchronization are used. Virus can be isolated from the urine and feces of kittens surviving experimental in utero inoculation at 3 and 6 weeks after birth, respectively. Using PCR, attenuated FPV has been detected in tissues for at least 19 days after vaccination.19 Direct culture from trypsinized lung and kidney tissues allows improved isolation of the virus for up to 70 days. Virus has been isolated by direct culture for up to 1 year from the lungs and kidneys of prenatally infected kittens, despite a high level of circulating antibody. Virus can be found in the CNS for at least 22 days after neonatal infection and thereafter persists in Purkinje’s cells. Direct fluorescent antibody testing can be used to detect virus in cell cultures and from tissues (usually intestine) of infected cats within 2 days after infection. Monoclonal antibodies can be used to distinguish FPV from CPV strains, as can PCR followed by restriction enzyme digestion analysis.2,46,46 PCR has been used to identify FPV in whole blood, fecal, intestinal, and tissue samples from cats.* Blood testing is used when cats do not have diarrhea or available feces for testing. Genetic detection methods are especially valuable when viral quantities are low, because these specimens will have negative test results with immunoassay procedures. Because of greater test sensitivity, virus can be identified for longer periods using viral isolation or genetic methods as compared to ELISA methods. Detection of virus by genetic methods may be too sensitive in some cases, and subclinical shedders will be found. MLV vaccination of cats can also produce false-positive results on fecal antigen testing.97 More perplexing was that certain test kits yielded positive results following use of inactivated vaccine, which could have been caused by exposure to MLV shedding of contact cats or inherent false-positive results in the test system.97 Positive test results should always be interpreted with respect to recent MLV vaccination, compatible clinical signs, and hematologic alterations. Genetic detection methods also can be used on formalin-fixed, paraffin-embedded tissues. Because carnivore parvoviruses can cross-infect multiple species, genetic detection permits specific identification of the viral strain. Gross pathologic changes in naturally infected cats are usually minimal. Focal ulceration may be found on the surface of the tongue. The intestinal tract is obviously dilated; the bowel loops are firm and may be hyperemic (Fig. 9-4) with petechial and ecchymotic hemorrhages on the serosal surfaces. The feces frequently have a fetid odor when blood is present. Prenatally infected cats may have a small cerebellum, hydrocephalus, or hydranencephaly (Fig. 9-5). Thymic atrophy, present in all infected neonates, is the only gross finding in germ-free kittens.
Feline Enteric Viral Infections
Feline Parvovirus Infection
Etiology
Hosts
Monoclonal Antibody Reactivity
Nucleotide Position vp2 Gene
Virus
Year of Origin
Natural Host
Experimental Host/Tissue of Replication
Cell Culture Lines
A3B10
B6D5
B2G11
B4E1
A4E3
C1D1
B4A2
P2–215
239
246
259
279
308
699
871
889
899
913
967
1038
1167
1276
1623
1691
1703
FPV/MEV-1
<1900 1940
Cat, mink, raccoon
Dog/only thymus, marrow
Feline only
++
++
++
++
−
−
++
++
A
A
A
A
T
T
T
C
C
G
G
A
T
A
A
A
C
BFPV/MEV-2
1983
Blue fox, mink, raccoon
Feline only
Feline only
−
++
−
−
−
−
++
++
A
A
A
A
T
T
C
C
T
G
G
G
C
A
A
A
C
CPV-2
1978
Dog, raccoon dog
Cat/no replication
Feline, canine
++
++
+
++
++
−
++
−
G
G
A
C
C
C
C
C
C
G
A
G
C
A
A
G
G
CPV-2a
1981
Dog, cat
Cat/lymphoid intestinal
Feline, canine
++
++
−
−
++
++
++
−
G
G
T
C
C
C
C
G
G
T
A
G
C
A
C
G
G
CPV-2b
1985
Dog, cat
Cat/lymphoid intestinal
Feline, canine
++
++
−
−
++
++
−
−
G
G
T
C
C
C
C
G
G
T
A
G
C
G
C
G
G
CPV-2c (a)
1997
Leopard cat
Cat? Dog?
Feline, canine
−
−
−
−
++
−
++
−
G
G
T
C
C
C
C
G
A
T
A
G
C
A
C
G
G
CPV-2c (b)
1997
Leopard cat
Cat? Dog
Feline, canine
−
−
−
−
++
−
−
−
G
G
T
C
C
C
C
G
A
T
A
G
C
G
C
G
G
Epidemiology
Pathogenesis
Systemic Infections
Co-Infections
In Utero Infection
Central Nervous System Infection
Myocarditis and Cardiomyopathy
Clinical Findings
Diagnosis
Clinical Laboratory Findings
Serologic Testing
Fecal Viral Antigen Testing
Viral Isolation
Genetic Detection
Pathologic Findings
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Feline Enteric Viral Infections