Fig. 2.1
Scutal patterns of female of Aedes aegypti and Ae. albopictus

Fig. 2.2
Female of Aedes albopictus
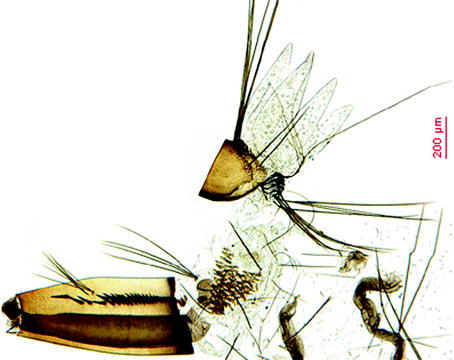
Fig. 2.3
Larva of Oc. j. japonicus
Larva: The antennal seta (1-A) is small and single, inserted slightly beyond the middle of the antennal shaft. The frontal setae (5-C to 7-C) are long and single, and the outer frontal seta (7-C) very rarely has two branches. The comb consists of a single irregular row of 6–12 scales, each scale with a long median spine and strong subapical spines forming a “trifid” appearance. The pecten has 8–22 teeth (usually 10–16), evenly spaced, or sometimes the distal most tooth is detached apically. The siphonal tuft (1-S) has three or four branches, usually inserted close to the distal pecten tooth and just beyond the middle of the siphon.
Biology: The “African tiger mosquito” (“yellow fever mosquito”), Ae. aegypti, is supposed to have its origin in Africa. An ancestor form (Ae. aegypti formosus) is still present on the African continent, whereas a domestic form (Ae. aegypti aegypti) has spread across almost all tropical and subtropical countries, during the past five centuries.
Its populations increase especially in areas where household water storage in containers is common and where solid waste disposal services are inadequate. In subtropical climates, the species is found almost always in the close vicinity of human settlements. The larvae occur in a wide variety of small artificial containers and water recipients of all kinds, both inside and outside of human habitations in gardens and within a circle of 500 m around dwellings, for example, in earthenware pots and water tanks for storing water, uncovered cisterns, rain-filled empty cans or flower pots, broken bottles, or discarded tires. The larvae may also be found in tree holes, leaf axils, bamboo stumps, or coconut shells after heavy rainfall. The breeding water is mostly clean or has a moderate content of organic matter. The larvae spend a long time under water feeding on the bottom of their breeding sites. The eggs are resistant to desiccation and are deposited close to the waterline in the mentioned recipients. At a temperature of 27–30°C, the larvae will hatch 2 days after egg deposition if flooded, pupation occurs after 8 days, and the adults emerge from the pupae 9–10 days after the eggs have been flooded. The females feed predominantly during the day in shaded places and only occasionally during the night in rooms. Human blood seems to be preferred to that of domestic animals (Carpenter and La-Casse 1955). The adults are frequently found resting indoors; they do not migrate over long distances and rarely fly more than several 100 m from their breeding sites. The available literature about Ae. aegypti, its biology, and medical significance is numerous, and a well-recognized monograph was published by Christophers in 1960.
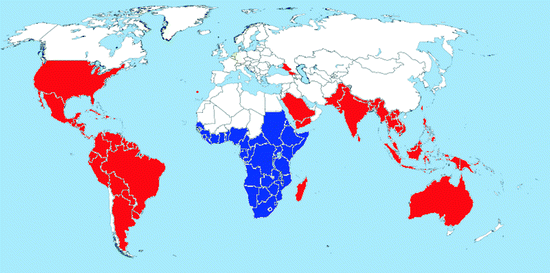

Map 2.1
Worldwide distribution of Ae. aegypti (blue = countries with native populations; red = countries with nonnative (established) populations; yellow = countries with sporadic occurrence – not established populations)
Distribution: This cosmotropical species is distributed in the tropical, subtropical, and warm temperate regions of both hemispheres (Map 2.1). Its range is mainly limited by the 10°C cold-month isotherms allowing continuous breeding all year round (Christophers 1960). Certain populations may extend their summer range considerably north of this line, for example, in North America, specimens may be found up to 40° northern latitude in southern Illinois and Indiana, but they are not able to survive during cold winter months; this prevents the establishment of permanent populations. In Europe, prior to 1945, all Mediterranean countries and most major port cities had reported at least occasional introductions of Ae. aegypti (Mitchell 1995). It could be found in Portugal, Spain, France, the UK, Italy, former Yugoslavia, Greece, Turkey, Ukraine, and Russia, but has been eradicated from these countries. Recently, Ae. aegypti reestablished in Madeira and around the Black Sea in southern Russia, Abkhazia, and Georgia (Riabova et al. 2005; Almeida et al. 2007). Very recently, Ae. aegypti was even found in the Netherlands in used tires imported from the USA, associated with Ae. atropalpus and Ae. albopictus (Scholte et al. 2010). This is the first record of Ae. aegypti in northern Europe after decades (Hansford et al. 2010).
Medical importance: As the major vector of yellow fever virus, Ae. aegypti has long been notorious as the “yellow fever mosquito,” but it is also an important vector of dengue, chikungunya, and several other viral infections.
2.3.2 Aedes (Stegomyia) albopictus (Skuse) 1894
[= Stegomyia albopicta sensu Reinert et al. 2004]
Female: The scutum is mainly covered with narrow dark scales, with a prominent longitudinal stripe of narrow white scales in the middle of the scutum (Figs. 2.1 and 2.2). The hind tibiae are entirely dark, hind tarsus has broad basal white bands on tarsomeres I–IV, and tarsomere V is all white.
Larva: The antennal seta (1-A) is simple and small and situated close to the middle of the antennal shaft. The median frontal seta (6-C) has 1–2 branches, and the inner frontal seta (5-C) is longer and simple. The comb consists of 6–13 (usually 8–10) long slender scales in a single row. The siphon is short and tapers distinctly from the middle; the siphonal index is 1.7–2.5. The number of pecten teeth is between 8 and 14; they are evenly spaced. The siphonal tuft (1-S) has 2–4 branches and is inserted beyond the distal most pecten tooth, slightly beyond the middle of the siphon.
Biology: The immature stages occur in a variety of small natural and artificial containers, for example, in tree holes, bamboo stumps, coconut shells, rock holes, plant axils, or palm fronds and in flower pots, tin cans, water jars, metal and wooden buckets or drums, broken glass bottles, or discarded tires (Huang 1972; Becker et al. 2010). The eggs are resistant to desiccation which facilitates their transport in used tire casings, even over long distances. Continuous breeding throughout the year takes place in tropical and subtropical areas, but in more temperate climatic zones, such as Europe, populations of Ae. albopictus are found which show embryonic diapause and overwinter in the egg stage. Several generations per year may occur. Adult females predominantly feed on humans but may also bite other mammals including rabbits, dogs, cows, and squirrels or occasionally avian hosts. This feeding behavior indicates that Ae. albopictus is well suited for transmitting of a variety of arboviruses that use mammals and birds as their main hosts (Mitchell 1995). To feed on humans, the females bite during daytime outside houses, mainly in shaded situations, and rarely enter dwellings during dusk and night (Delatte et al. 2010). Aedes albopictus is a mass species in East Asia causing great nuisance wherever it occurs and, although not present before 1990, has become a major pest species in some areas of the USA and Italy.
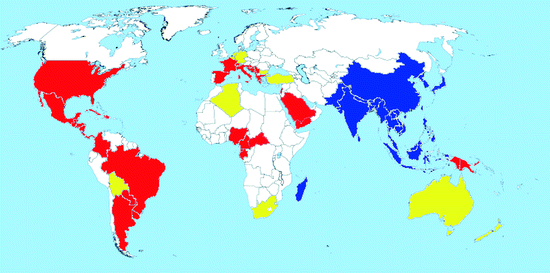

Map 2.2
Worldwide distribution of Aedes albopictus (blue = countries with native populations; red = countries with nonnative (established) populations; yellow = countries with sporadic occurrence – not established populations)
Distribution: The Asian tiger mosquito Ae. albopictus, originating from Southeast Asia, has undergone a noteworthy expansion of its range within few decades (Hawley 1988). With the increase in international trade of used tires, Ae. albopictus has spread across very large distances and between continents (Reiter and Sprenger 1987; Reiter 1998; Scholte and Schaffner 2007).
The mosquito spread from its native range initially to the Pacific Islands (Gratz 2004) in the 1930s and then, by the end of the century, to other countries in both the Old and New World (Moore and Mitchell 1997; Gratz 2004). In the Palaearctic, it occurred in Japan and China. In 1985, it was discovered for the first time in the New World (Houston, Texas), and this was the beginning of a rapid spread of Ae. albopictus on the American continent as well as new introductions in many parts of the world (Mitchell 1995). It is now present in over 25 states of the USA and in several countries of South America and Africa (Map 2.2). Specimens have been found in Australia and New Zealand, but breeding populations have so far not become established there.
In Europe, Ae. albopictus has probably been present in Albania since at least 1979 (Adhami and Murati 1987; Adhami and Reiter 1998). In the early 1990s, it was passively introduced into Italy due to the international trade of used tires. The species was first detected in Genoa in September 1990 (Dalla Pozza and Majori 1992) followed by a rapid spread into other areas of northern and central Italy (Romi 1994). Since 1999, Ae. albopictus has been found in various southern and central European countries, including France (Schaffner et al. 2001), Bosnia and Herzegovina (Petrić et al. 2006), Montenegro (Petrić et al. 2001), Belgium (Schaffner et al. 2004), Switzerland (Flacio et al. 2004), Greece (Samanidou-Voyadjoglou et al. 2005), Malta (Gatt et al. 2009), Monaco (ECDC 2009), Croatia (Klobucar et al. 2006), San Marino (ECDC 2009), Slovenia (Petrić et al. 2006), Spain (Aranda et al. 2006), the Netherlands (Scholte et al. 2007), Vatican City (ECDC 2009), and Germany (Pluskota et al. 2008; Werner et al. 2012).
Another way of spreading the eggs and larvae of Ae. albopictus is the trade with the ornamental plant Dracaena sp. (“lucky bamboo”). These plants are packaged in standing water during shipment and permit an “ideal insectary in transit” which led to the introduction of Ae. albopictus from Asia to California and the Netherlands (Madon et al. 2004; Scholte et al. 2007). The species is not yet established in Belgium and Germany neither in the Netherlands where it is repeatedly introduced and controlled.
Medical importance: Aedes albopictus is a vector of at least 22 arboviruses, including chikungunya, West Nile, dengue, and yellow fever viruses as well as Dirofilaria immitis (dog heartworm) (Gubler and Rosen 1976; Gratz 2004; Becker et al. 2010). Although Ae. aegypti is the principal vector of dengue fever, recent outbreaks of dengue disease in the absence of Ae. aegypti have implicated Ae. albopictus as a competent vector (Gratz 2004; Effer et al. 2005). It is assumed that the Asian tiger mosquito is also involved in the autochthonous transmission of Dengue viruses in Europe, namely, in southern France and Croatia as well as in the outbreak of chikungunya fever in Italy 2007 (Bonilauri et al. 2008; La Ruche et al. 2010; Schmidt-Chanasit et al. 2010; Gjenero-Margan et al. 2011).
2.3.3 Ochlerotatus (Finlaya) japonicus japonicus (Theobald 1901)
[sensu Reinert et al. 2004 = Aedes japonicus sensu auctorum = Hulecoeteomyia japonica sensu Reinert et al. 2006]
There exist at least four morphologically similar subspecies (Tanaka et al. 1979) which occur in different parts of East Asia: Oc. japonicus japonicus (Palaearctic Japan and Korea), Oc. japonicus shintiensis (Taiwan), Oc. japonicus yaeyamensis, and Oc. japonicus amamiensis (both on the Ryukyu Archipelago). The subspecies differ by morphology mainly in the ornamentation of the hind femora.
Female: The adult female of Oc. japonicus is a medium/large-sized mosquito of dark to blackish-brown appearance, with white scales on the body and legs. It is similar to the closely related Oc. koreicus. The pattern on the scutum is distinctive with five golden stripes of scales; the lateral scales are pale and white in a lyre shape. There are also broad pale basal bands on the hind tarsomeres I–III, and tarsomeres IV and V are entirely black. Subspiracular area bears only a few scales (contrary to Oc. koreicus). The proboscis is entirely dark. The head is dark and the abdomen is creamy colored (Miyagi 1971). Terga II–VII dark scaled with lateral patches of white scales at the base of each segment.
Larvae: Typical are the pecten with detached very strong teeth and the anal saddle with its highly spiculated distal margin; the inner and median frontal setae are multiple (tufts) and arranged in a straight line close to the apical margin of the head. The siphonal tuft has multiple branches (4–6), is very short, and inserted at the middle of the siphon and within the pecten. Comb scales are displayed in a patch, and the siphon index is 2.5 (Fig. 2.3).
Biology: Adult of Oc. j. japonicus prefer forested areas and bite usually during the daytime (Tanaka et al. 1979). They feed on a great variety of mammals and birds such as dogs, pigs, deers, rodents, or chicken (Scott 2003). They are more reluctant to bite humans and not as aggressive biters as floodwater mosquitoes like Ae. vexans or Oc. sticticus.
This multivoltine species has adapted to colder climates and can survive in their desiccation-resistant eggs even during cold winter times (Tanaka et al. 1979; Andreadis et al. 2001). Under moderate climate conditions, they can also be found throughout the winter as larvae. The larvae of Oc. japonicus are typically found in a great variety of usually small-volume natural water collections such as tree holes, bamboo stems, or rock pools or in artificial breeding sites including vases, tins, tires, drums, buckets, roof gutters, cement catch basins, or bird baths. They prefer breeding sites which are rich in organic matter, but not heavily polluted.
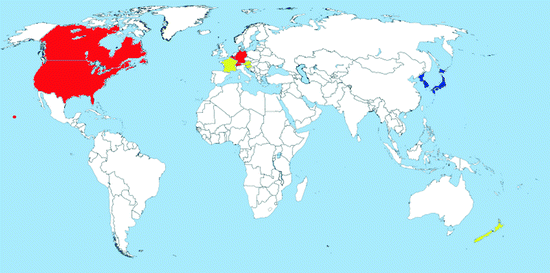

Map 2.3
Distribution of Oc. japonicus (blue = countries with native populations; red = countries with nonnative (established) populations; yellow = countries with sporadic occurrence – not established populations)
Distribution: Ochlerotatus japonicus is an Asian species originally found in Japan, Korea, South China, Taiwan, and the eastern part of the Russian Federation (Tanaka et al. 1979). It was intercepted several times in New Zealand in the 1990s (Laird et al. 1994; Fonseca et al. 2001). It has established for the first time outside its native range in the USA in 1998; it is now recorded in at least 29 other states of the country including Hawaii as well as in Canada (Map 2.3) (Andreadis et al. 2001; Saenz et al. 2006; Williges et al. 2008; Fonseca et al. 2010). The species was introduced via international transport mostly of used tires (Peyton et al. 1999; Thielman and Hunter 2006).
In 2000, Oc. j. japonicus was first recorded in Europe when larvae were found on a storage yard of imported used tires in France (Schaffner et al. 2003), from where it was successfully eradicated, thanks to immediate control measures. Since 2002, Oc. j. japonicus has repeatedly been observed on a secondhand tire company in southern Belgium (Versteirt et al. 2009a, b). Finally, in 2008, the species was detected in northern Switzerland spreading into bordering Germany (Schaffner et al. 2009). Since 2009, an intensive monitoring program of the distribution of Oc. j. japonicus has been conducted in Southern Germany (state of Baden-Württemberg). More than 6,500 water-filled containers in 291 municipalities distributed over the whole area of Baden-Württemberg have been investigated for Oc. j. japonicus developing stages. Out of 291 municipalities, 54 (18, 2%) were positive for Oc. j. japonicus covering an area of about 2,000 km2 (Becker et al. 2011; Huber et al. 2012). Recently, a second focus localized 80 km apart from the known colonized area was reported (Schneider 2011). Finally, the species was reported from a large cross-bordering area of Austria and Slovenia (B. Seidel, unpublished).
Medical importance: Ochlerotatus j. japonicus is a competent laboratory vector of several arboviruses, such as West Nile (WN) virus and Japanese encephalitis (JE) virus, but it can also transmit St. Louis encephalitis, Eastern equine encephalitis, La Crosse, Dengue, and Chikunguny viruses (Schaffner et al. 2011) and is considered a significant public health risk (Sucharit et al. 1989; Sardelis and Turell 2001; Sardelis et al. 2002a, b, 2003).
2.3.4 Ochlerotatus (Finlaya) koreicus (Edwards 1917)
[sensu Reinert et al. 2004 = Aedes koreicus sensu auctorum = Hulecoeteomyia koreica sensu Reinert et al. 2006]
Females: The midscutal line is broad, and the submedian line is broken at the level of the scutal angle, the anterior end of the posterior part extending outward along the scutal angle to the scutal margin. The hind tarsi I–IV are basally banded, and the fifth tarsus bears some pale scales or even a basal band (Oc. japonicus: only hind tarsi I–III basally banded). The subspiracular area bears a distinct patch of broad white scales (Tanaka et al. 1979).
Larvae: The larva reminds those of Oc. japonicus but has no enlarged simple pecten tooth nor a spiculated distal margin of the anal saddle. The apical most teeth may be a little bit larger than the others, but never detached, as in Oc. japonicus (Miyagi 1971).
Biology: Adults bite during day and night and suck blood on humans and mammals. Immature stages can be found in natural breeding sites such as rock pools and tree holes but also in a great variety of artificial containers such as used tires or vases. The species overwinters in the egg stage, and the larvae are hatching after the snowmelt.
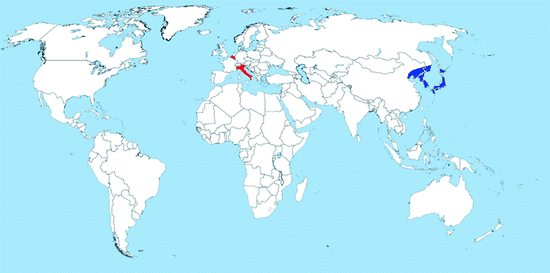

Map 2.4
Distribution of Oc. koreicus (blue = countries with native populations; red = countries with nonnative (established) populations; yellow = countries with sporadic occurrence – not established populations)
Distribution: Ochlerotatus koreicus is an Asian species native to Japan, China, Korea, and eastern Russia (Map 2.4). It was first found outside of its native range in Belgium in 2008 (Versteirt et al. 2009a, b). Very recently, in May 2011, developmental stages of Oc. koreicus were collected in the Veneto region from a manhole and other artificial containers (Capelli et al. 2011). The species is tolerant to cold climates, making it capable of surviving and becoming established in Belgium and the hilly and pre-alpine areas of Italy. It seems that after Oc. japonicus, this species is starting to spread across the world (Cameron et al. 2010). No introduction route could be evidenced so far, neither direct from Asia nor from Belgium to Italy.
Medical importance: The species is considered a potential vector of arboviruses. It has been shown experimentally to be able to transmit Japanese encephalitis virus and the dog heartworm Dirofilaria immitis. However, vector competence studies are strongly required to better define the role of this mosquito in the transmission of arboviruses, such as West Nile and USUTU viruses (Capelli et al. 2011).
2.3.5 Ochlerotatus (Ochlerotatus) atropalpus (Coquillett 1902)
[sensu Reinert et al. 2004 = Aedes atropalpus sensu auctorum = Georgecraigius atropalpus sensu Reinert et al. 2006]
Females: Scutal scales are pale yellow, dark scales are mixed with light scales, and sometimes a darker medial stripe is present. Terga II–VII have narrow white basal bands of scales and dark brown apically. The hind tibia has pale scales at basis and apex. The hind tarsus has large basal and smaller apical ring of whitish scales at each tarsal joint; hind tarsomere V is entirely white (Wood et al. 1979).
Larvae: Ochlerotatus atropalpus resembles Oc. japonicus based on their detached pecten teeth and the siphonal tuft inserted within the pecten row; however, it can be separated by their inner and median frontal setae which are single and the difference in spiculation on the anal saddle. The pecten extends almost to the tip of the siphon, and the siphonal tuft inserts at the middle of the siphon within the row of pecten teeth. The comb scales are arranged in patch-like, irregular rows, contrary to Ae. aegypti, Ae. albopictus, and Oc. triseriatus with comb scales in a row (Wood et al. 1979).
Biology: Ochlerotatus atropalpus is a multivoltine species with a life cycle that resembles Ae. triseriatus in terms of larval habitats. It is most often associated with fresh water rock pool habitats along mountain streams in Northern America’s inland areas (common name: American rock pool mosquito). However, it is known to breed in a variety of artificial containers especially in discarded tires and other man-made water collections such as in boats (King et al. 1960; Shaw and Maisey 1961; Berry and Craig 1984). The species bites human and other mammals outdoor during daytime.
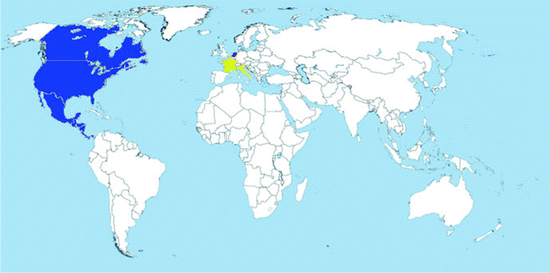

Map 2.5
Worldwide distribution of Oc. atropalpus (blue = countries with native populations; red = countries with nonnative (established) populations; yellow = countries with sporadic occurrence – not established populations)

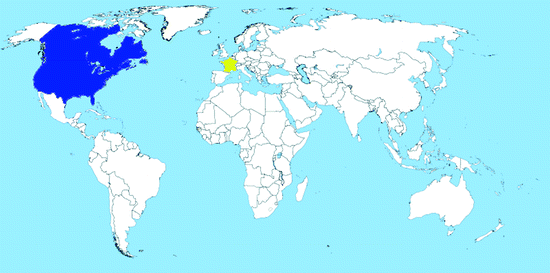
Map 2.6
Worldwide distribution of Oc. triseriatus (blue = countries with native populations; red = countries with nonnative (established) populations; yellow = countries with sporadic occurrence – not established populations)
Distribution: The species is native from North and Central America. It is found from Labrador, Canada to Panama, and from the Atlantic seaboard to Baja California (O’Meara and Craig 1970).
In Europe, it has been recorded from Italy (1996), France (2004), and the Netherlands (2009) (Map 2.5). In all cases, it was introduced via trade of used tires (Romi et al. 1997; Scholte and Schaffner 2007; Scholte et al. 2009).
2.3.6 Ochlerotatus (Protomacleaya) triseriatus (Say 1923)
[sensu Reinert et al. 2004 = Aedes triseriatus sensu auctorum]
Female: Proboscis and palps dark scaled. Scutum with a middorsal longitudinal broad stripe of brown scales. Two stripes of silvery scales on the dorsal side of the thorax are the most diagnostic characteristic (Wood et al. 1979). The abdominal segments are dark and unbanded. Most of the tibiae and all tarsi are dark scaled (unbanded).
Larvae: Larvae have a characteristic gray coloration and swim with a characteristic serpentine motion. Frontal setae 5-C are single, and 6-C are 2–3 branched. The pecten teeth are evenly spaced, and the siphonal tuft inserts beyond the uppermost tooth (Wood et al. 1979). The comb has a single row of scales that are arranged in an extremely irregular fashion (comb scales of Ae. aegypti and Ae. albopictus are set in a concise single row). The ventral pair of anal gills is shorter than the dorsal pair (Ae. albopictus equal in size).
Biology: Oc. triseriatus is a multivoltine species which overwinters in the egg stage. The typical breeding sites are tree holes (common name: American eastern tree hole mosquito), but developmental stages can be found also in artificial breeding sites such as discarded tires, buckets, barrels, and cans. The eggs can withstand long periods (a year or more) without water. The females bite during day, especially in shaded areas. They take blood meals from humans, other mammals, and even birds. In tire yards, it can become a major pest species. However, the flight range is only a few 100 m from the breeding area (Wood et al. 1979).
Distribution: Ochlerotatus triseriatus is widely distributed in Canada and the USA (east of the Rocky Mountains) and inhabits all of the Southeastern United States, from Minnesota and Texas to Maine and Florida (Map 2.6). The species was found at one occasion in Greenland, maybe after accidental introduction (Messersmith 1971); it is not known if the species has persisted there. More recently, some females were intercepted at a used tire yard in France in 2004, freshly imported from the USA; immediate control measures prevent the species to establish (F. Schaffner in Medlock et al. 2012).
Medical importance: It can transfer a variety of viruses such as eastern equine encephalitis and is linked to the spread of La Crosse encephalitis virus (Watts et al. 1973).
2.4 Surveillance of Exotic Mosquitoes
The primary dispersal mode of exotic mosquitoes by human activity is through transport of desiccation-resistant eggs in cargo that previously contained stagnant water. The most important type of cargo is used tires that have been stored outdoors (Knudsen 1995). Businesses processing or trading used tires should be given high priority for monitoring populations. Another source of introduction is by ornamental plants, for example, “lucky bamboo” (Dracaena spp.) from Southeast Asia. For instance, “lucky bamboo,” which is transported in containers with standing water, permits an “ideal insectary in transit.” Thus, multiple introductions of the “Asian tiger mosquito” to the Netherlands in greenhouses of horticulture companies could be traced back to intensive trade of this plant (Scholte et al. 2007, 2010).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


