Chapter 34 Exotic Mammal Diagnostic and Surgical Endoscopy
With over 10 million pet rabbits (Oryctolagus cuniculus), ferrets (Mustela putorius furo), and rodents (order Rodentia) in the United States, these exotic mammals represent the third largest group of companion mammals (behind dogs and cats).1 This group represents an expanding component of small-animal practice, with many clients expecting the same level of medicine for them as for our more traditional clientele. The advent of the certifying examination in zoological companion species (American College of Zoological Medicine) and exotic companion mammals (American Board of Veterinary Practitioners) has further galvanized the need for veterinarians to provide the expected high-quality care being demanded for these species. The majority of rabbits, ferrets, and rodents presented to practitioners weigh less than 2 kg, and, given their small size, are ideal candidates for minimally invasive diagnostic and surgical endoscopy. Indeed, in some situations, the development of endoscopy has enabled many procedures to be performed for the first time or with significantly reduced morbidity and mortality compared with traditional surgery. Considerable advances in exotic animal endoscopy have been made over the past 5 years, and further development and refinement seems assured.8
Anesthesia
The diversity within the exotic mammal group necessitates generalities rather than specifics, and reference should be made to the extensive literature on rabbit, ferret, and rodent anesthesia for precise drug dosages.3–5 Ferrets are typically fasted for 6 to 8 hours. Rabbits and rodents seldom vomit or regurgitate and are fasted (which includes removal of food and all bedding materials) for only 1 to 2 hours to reduce material within the oral cavity. An opioid-benzodiazepine premedicant is generally effective and, following preoxygenation by mask, induction can be accomplished using intravenous or intramuscular ketamine alone or in combination with an additional benzodiazepine (rabbits and rodents), or intravenous propofol (ferrets). Unlike ferrets, in which it is relatively easy to place an endotracheal tube, rabbits and rodents are more challenging, and ketamine provides sufficient time for visualization, application of a local anesthetic, and careful visual intubation. Small rodents that cannot be intubated are best induced and maintained using isoflurane or sevoflurane with oxygen via a close-fitting rodent mask and dedicated nonrebreathing rodent circuit. Maintain animals on isoflurane or sevoflurane in oxygen adjusted to individual patient requirements. Ventilation is often required, especially in rabbits and rodents in dorsal recumbency, because of gastrointestinal pressure on their diaphragms, reducing ventilation. However, ventilation is essential for all laparoscopy, thoracoscopy, and procedures requiring neuromuscular blockade (e.g., rhinoscopy). Hypothermia is difficult to prevent unless warm-water- and air-circulating blankets and drapes or covers are used. Warming air filters attached to the endotracheal tube can also be useful for maintaining temperature, but these often require ventilator support. In addition to reflexes and muscle tone, anesthesia monitoring should include temperature, pulse, respiratory rate, end-tidal capnography, pulse oximetry, and direct or indirect blood pressure. Many of these aids become increasingly problematic as animal size decreases. During rhinoscopy of rabbits and larger rodents, intranasal lidocaine and short-term neuromuscular blockade using atracuronium further enhance immobilization; otherwise a much deeper plane of general anesthesia would be required.
Instrumentation
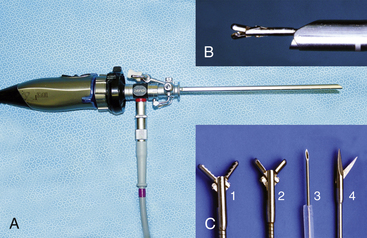
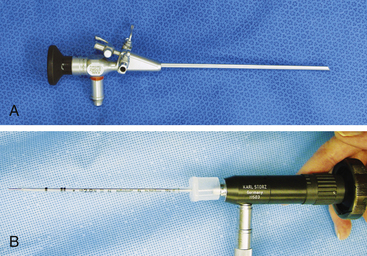
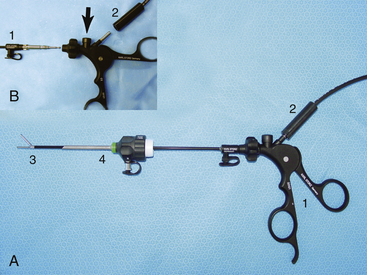
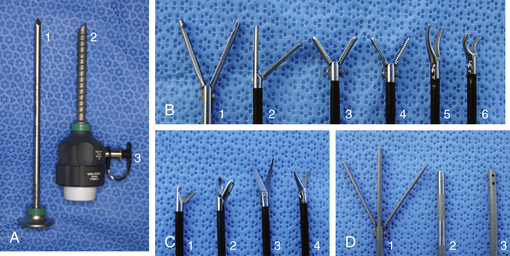
Given the variation in size and the nature of the procedures that may be performed, a variety of different scopes and instruments may be used (Table 34-1). For most practices the 2.7-mm system offers the greatest versatility, which can be built upon as individual practice caseload dictates. This system offers several advantages, including single-entry procedures, ports for air or saline infusion, and an operating channel for the introduction of 5-Fr instruments (Fig. 34-1). In addition, the 1.9-mm telescope with integrated sheath and the 1-mm semirigid miniscope are extremely useful for smaller mammals (Fig. 34-2). For multiple-entry endoscopy, the recent application of human pediatric 2- and 3-mm instruments to exotic animal endoscopy has enabled laparoscopy and thoracoscopy to become a reality (Figs. 34-3 and 34-4).6
Table 34-1 Endoscopic Instrumentation for Ferrets, Rabbits, and Rodents
| Equipment Description | Primary Indications |
|---|---|
| Visualization & Documentation | |
| Endovideo camera and monitor Xenon light source and light guide cable Digital capture device (e.g., AIDA-Vet) | Required for all endoscopy procedures |
| Endoscopes | |
| 1-mm x 20-cm semirigid miniscope, 0° | Stomatoscopy, otoscopy, rhinoscopy in animals <500 g Endotracheal intubation in animals <1 kg |
| 1.9-mm x 18.5-cm telescope, 30° oblique, with integrated 3.3-mm operating sheath | Stomatoscopy, otoscopy, rhinoscopy in animals 500 g-10 kg Endotracheal intubation in animals 1-8 kg Laparoscopy in animals <1 kg |
| 2.7-mm x 18-cm telescope, 30° oblique 4.8-mm operating sheath | Stomatoscopy, otoscopy, rhinoscopy in animals 500 g-10 kg Endotracheal intubation in animals 1-8 kg Laparoscopy and thoracoscopy in animals 1-8 kg |
| 5-mm x 8.5-cm otoendoscope, 0°, with integrated operating sheath | Stomatoscopy and otoscopy in animals 3-40 kg |
| Flexible Instruments for Use with Operating Sheaths | |
| 1-mm biopsy forceps 1-mm grasping forceps | For use with 1.9-mm telescope and integrated sheath |
| 1.7-mm x 34-cm biopsy forceps 1.7-mm x 34-cm single-action scissors 1.7-mm remote injection needle 1.7-mm x 34-cm grasping/retrieval forceps 1.7-mm x 60-cm wire basket retrieval 1.7-mm needle end radiosurgery electrode | For use with 2.7-telescope and 4.8-mm operating sheath, and 5-mm otoendoscope |
| Insufflation | |
| CO2 insufflator with silicone tubing | Used for insufflation during laparoscopy |
| Sterile saline suspended above endoscopy table with intravenous drip line to a port on the operating sheath | Used for sterile saline infusion for otoscopy, rhinoscopy, cystoscopy, vaginoscopy, and colonoscopy |
| Rigid Instruments and Cannulas for Multiple-entry Laparoscopy and Thoracoscopy | |
| 2.5-mm graphite and plastic cannula 2-mm Reddick-Olsen dissecting forceps, plastic handle without racket 2-mm Metzenbaum scissors, plastic handle without racket 2-mm Babcock forceps, plastic handle with racket | Used with the 1.9-mm telescope for laparoscopy and thoracoscopy in animals <1 kg |
| 3.9-mm graphite and plastic cannula (accommodates 2.7 mm telescope and 3.5-mm protection sheath) 3.5-mm graphite and plastic cannula (accommodates 3-mm instruments) 3-mm fenestrated grasping forceps 3-mm Reddick-Olsen dissecting and grasping forceps 3-mm short curved Kelly dissecting and grasping forceps 3-mm atraumatic dissecting and grasping forceps 3-mm Babcock forceps 3-mm Blakesley dissecting and biopsy forceps 3-mm scissors with serrated curved double action jaws 3-mm micro-hook scissors, single action jaws 3-mm Mahnes bipolar coagulation forceps 3-mm irrigation and suction cannula 3-mm palpation probe with centimeter markings 3-mm distendable palpation probe 3-mm ultramicro needle holder 3-mm knot-tier for extracorporeal suturing 2 plastic handles without rackets 1 plastic handle with Mahnes-style racket 1 plastic handle with hemostat-style racket | Used with the 2.7-mm telescope for laparoscopy and thoracoscopy in animals 1-8 kg |
| Radiosurgery Equipment | |
| 3.8- or 4.0-MHz dual radiofrequency unit with foot pedal Monopolar lead to connect to plastic instrument handles Bipolar lead to connect to 3-mm Mahnes bipolar coagulation forceps | Enables endoscopic instruments to be used as monopolar devices and facilitates bipolar coagulation |
Procedures
In general, the approach to exotic mammal endoscopy is similar to that in domesticated dogs and cats, and much can be learned and applied from the domestic animal and human literature.10,12 However, in addition to the anatomic peculiarities of these creatures, the exotic mammal endoscopist must be more precise, given the confines within which it is often necessary to work. It is therefore particularly important to use the fingers and thumb of the inferior hand to support the tip of the telescope in order to ensure accurate control at all times.
Otoscopy
With the anesthetized animal in sternal or lateral recumbency, a detailed evaluation of the ears can be undertaken. In cases of severe disease, gently remove superficial exudates and debris before employing sterile saline infusion and working within a fluid environment. Abnormalities can be sampled for histopathology and microbiology using the biopsy forceps, which tends to provide more precise results than introducing a culturette down the vertical canal. Depending upon the size of the animal, diameter of the scope, and nature of the aural disease, it is often possible to examine the vertical and horizontal canals down to the tympanum (Fig. 34-5). It is especially important to examine the tympanum in rabbits and rodents as head tilt due to otitis media or interna is common. In ferrets, ear mites and aural neoplasia appear to be more common than bacterial infection.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree