Elizabeth A. Carr To evaluate a horse with urinary tract disease, the clinician should collect a complete history and perform a thorough physical examination. Important aspects of the history include information pertaining to the diet, medications administered, response to treatment, number of horses affected, and duration and type of clinical signs. A history of repeated nonsteroidal antiinflammatory drug (NSAID) use or recurrent rhabdomyolysis would increase the suspicion of renal disease. Water intake and urinary output should also be assessed. For example, owners may mistake pollakiuria, frequent urination, for polyuria, increased urine production. Differentiating between these two conditions is helpful in forming a diagnostic plan. Pollakiuria is frequently seen in females during estrus, and is seen with cystic calculi or cystitis in both sexes. In contrast, polyuria more commonly accompanies renal disease, diabetes insipidus, diabetes mellitus, pituitary pars intermedia dysfunction, and behavioral problems, such as psychogenic water drinking or salt eating. Astute owners may observe increased thirst following exercise or a change in urine appearance, such as a clearer stream, to support the presence of polydipsia and polyuria. Clinicians can determine water intake during a 24-hour period by turning off automatic watering devices and providing a known volume of water to the horse. Water intake may vary widely with diet, environmental conditions, and level of activity so that repeated measurements during several 24-hour periods may be more rewarding in documenting average daily water consumption. Horses stabled in a cool climate and fed a large quantity of the diet as a concentrate may drink as little as 15 to 20 liters of water daily, whereas horses exercising in hot climates have been recorded to drink as much as 90 liters daily. Urine output, which should range from 5 to 15 liters per day in a horse with normal renal function, is more difficult to determine. Urine collection harnesses can be applied for 24-hour urine collections; alternately, indwelling Foley catheters attached to a collection apparatus can be used to quantify urine output. The most common presenting complaints for horses with urinary tract disease are weight loss and abnormal urination. Other clinical signs vary with the etiology and location of the problem and may include colic, fever, inappetence, depression, ventral edema, oral cavity ulcers, excessive dental tartar, or scalding of the perineum or hind limbs. Colic may be reported in horses with urolithiasis and is often accompanied by hematuria, pollakiuria, and frequent straining to urinate. Although lumbar pain and hind limb lameness have been attributed to urinary tract disease, a musculoskeletal problem is the usual case of these clinical signs. A decrease in performance may be an early presenting complaint for renal disease, but poor performance is more likely a result of the mild anemia and lethargy that accompany uremia rather than a consequence of renal pain. In addition to a thorough physical examination, rectal palpation should be included in the evaluation of all horses with suspected urinary tract disease. The bladder should be palpated to determine size, wall thickness, and presence of cystic calculi or mural masses. If the bladder is full, palpation should be performed again after bladder catheterization or voiding because cystic calculi and masses may be missed during palpation of a distended bladder. The caudal pole of the left kidney can be palpated for size and texture in most horses. The ureters are not palpable unless they are enlarged or obstructed by disease. Ureteral dilatation may be assessed by palpation of their retroperitoneal course in the dorsal abdominal wall or through the vaginal wall in mares. Dilatation of a ureter may be detected in horses with pyelonephritis or ureteral calculi. The reproductive tract should also be palpated to assess whether a reproductive problem could be a possible cause of the clinical signs. Results of a complete blood count revealing a high white blood cell count and high protein or fibrinogen concentration support an inflammatory or infectious disease process. Mild anemia (indicated by a packed cell volume of 20% to 30%) consequent to decreased erythropoietin production and a shortened red blood cell life span may be found in horses with chronic renal failure. Blood urea nitrogen (BUN) and serum creatinine concentrations are the most commonly used indices to assess renal function, specifically glomerular filtration rate (GFR). Urea nitrogen is produced by the liver during protein metabolism and is affected by protein levels in the diet. Because the horse has a relatively constant low-protein diet, compared with carnivores’ diets, diet has little effect on BUN concentration. The concentration of BUN may increase with gastrointestinal bleeding and may decrease with liver dysfunction. It is lower in foals because of their high rate of protein synthesis. Urea is a highly filterable molecule that has variable reabsorption, depending on the volume of water excretion and electrolyte filtration. Despite the fact that most (90%) BUN is excreted by the kidney, it is considered a poor measure of GFR because of the fact that as much as half can be reabsorbed into the systemic circulation. Creatinine is produced in skeletal muscle, and serum concentrations will be higher in heavily muscled individuals. Similar to BUN, serum creatinine values are unaffected by the herbivore’s diet. Because creatinine is filtered by the glomerulus with little tubular reabsorption or secretion, serum creatinine levels correlate with GFR and can be useful in assessing changes in renal filtration. High creatinine concentrations have been measured in newborn foals without underlying renal disease. In utero placental insufficiency is suspected in these foals, and serum creatinine values generally decrease to normal within the first few days of life. Depending on the laboratory methodology being used, creatinine concentrations may be falsely elevated by endogenous chromogens, hyperglycemia, hyperproteinemia, and ketosis. Cephalosporin antimicrobial administration may also result in a false increase in serum creatinine concentration. In addition, hyperbilirubinemia may also result in falsely low creatinine concentrations. It is important to remember that increases in serum urea nitrogen and creatinine do not occur until approximately 75% of renal mass becomes nonfunctional. For example, complete loss of function in one kidney does not result in increases in BUN or creatinine concentration as long as contralateral renal function remains normal. These values are thus not very useful in evaluating early or minor changes in GFR. Once values have become high, however, small increases in serum urea nitrogen and creatinine are sensitive indicators of further deterioration in GFR because a doubling of BUN or creatinine can be interpreted as a 50% decline in remaining renal function. Azotemia is defined as an increase in nitrogenous compounds and may be renal, prerenal, or postrenal in origin. Prerenal azotemia is a result of decreased renal perfusion, whereas postrenal azotemia is a result of obstruction of the urinary tract. Therefore interpretation of serum chemistry test results should be made in light of hydration status and other presenting signs. Although specific threshold values for BUN and creatinine that differentiate renal disease from prerenal azotemia do not exist, the BUN-to-creatinine ratio can be suggestive of prerenal or renal azotemia. Creatinine is a charged molecule and is less membrane permeable than urea; therefore acute changes in renal function are more accurately reflected by changes in creatinine than in urea nitrogen, and the increase in creatinine is proportionately greater than the rise in BUN. This fact has led to use of the BUN-to-creatinine ratio to differentiate prerenal azotemia or acute renal failure from chronic renal failure. With prerenal azotemia, the BUN-to-creatinine ratio is generally higher because of reabsorption of urea nitrogen back into the systemic circulation. Similarly, with uroabdomen, serum BUN is higher because of its more rapid reabsorption across the peritoneal surface, compared with creatinine. In acute renal compromise, a BUN-to-creatinine ratio of less than 10 : 1 is expected, whereas the ratio should exceed 15 : 1 with chronic renal failure. Although the BUN-to-creatinine ratio may be useful to consider, the values are not always reliable, especially with chronic renal failure, in which BUN may vary considerably according to dietary protein intake. Measurements of urine concentration of creatinine (see following section on urinalysis) or the urine-to-serum creatinine ratio can provide useful information. Urine-to-serum creatinine ratios in excess of 50 : 1 (reflecting concentrated urine) are expected in horses with prerenal azotemia, whereas ratios of less than 37 : 1 were reported in a group of horses with primary renal disease. In addition to BUN and creatinine, serum electrolyte, protein (albumin and globulin), and glucose concentrations and muscle enzyme activities should be included in the laboratory database. Hypochloremia is the most consistent electrolyte abnormality seen in horses with polyuric renal failure. Hypochloremic metabolic acidosis can be seen in renal tubular acidosis. Affected individuals typically present with poor performance, weight loss, and inappetence, with two-thirds of horses having underlying renal disease. Hyponatremia has been variably reported in horses with renal disease and is most commonly found with urinary tract disruption and uroperitoneum. Serum potassium concentration is usually normal but may be high in horses with acute renal failure or uroperitoneum. Calcium and phosphorus concentrations vary in horses with renal disease. Hypercalcemia and hypophosphatemia are often found in horses with chronic renal failure, especially when fed alfalfa hay, whereas hypocalcemia and hyperphosphatemia are more common with acute renal failure. With protein-losing glomerulopathies, albumin tends to be lost to a greater extent than globulin because of the former’s lower molecular weight. Although low total protein and albumin concentrations can accompany chronic renal disease in many species, horses appear more refractory to development of hypoproteinemia and the nephrotic syndrome. In fact, some horses have an increase in globulin concentration, which suggests chronic antigenic stimulation associated with neoplasia, glomerulonephritis, pyelonephritis, or amyloidosis. Hyperglycemia (blood glucose >175 to 200 mg/dL) that results from stress, exercise, sepsis, pituitary pars intermedia dysfunction, or diabetes mellitus may result in glucosuria. When pigmenturia is a complaint, muscle enzyme activities are helpful in differentiating myoglobinuria from hematuria or hemoglobinuria. Urinalysis should be performed in all horses in which urinary tract disease is suspected. Urine can be collected midstream while the horse is voiding, by urethral catheterization, or by cystocentesis in foals. Color, clarity, odor, viscosity, and specific gravity should be evaluated at the time of collection. Normal equine urine is pale yellow to deep tan in color and is often turbid because of large amounts of calcium carbonate crystals and mucus. Urine appearance often changes during urination, especially toward the end of micturition, when more crystals tend to be voided. If pigmenturia or hematuria is seen, the clinician should note the timing and duration of discolored urine passage to help in localizing the source. Pigmenturia throughout urination is most consistent with myonecrosis or a bladder or kidney lesion, whereas passage of discolored urine at the start or end of urination is more commonly seen with lesions of the urethra or accessory sex glands. Urine specific gravity is a measure of the amount of solute in urine and is a useful estimate of urine concentration. In response to water deprivation, a horse with normal renal function should be able to produce concentrated urine with a specific gravity of 1.025 to 1.050. In contrast, foals typically have urine that is more dilute than serum (i.e., is hyposthenuric or has a specific gravity <1.008) consequent to a high-volume milk diet. Although the constant polyuria decreases their ability to generate a large osmotic gradient in the medullary interstitium, foals can produce urine with a specific gravity higher than 1.030 when dehydrated. With renal disease, the ability to produce either concentrated (specific gravity >1.025) or dilute (specific gravity <1.008) urine is lost. Thus, horses with chronic renal failure typically manifest isosthenuria, in which urine is produced that has an osmolality similar to that of serum (specific gravity of 1.008 to 1.014). In horses with dehydration or shock resulting from a number of problems, measurement of urine specific gravity can help differentiate prerenal from renal azotemia. A high urine specific gravity (>1.035) supports prerenal azotemia, whereas failure to concentrate urine in the face of dehydration supports a diagnosis of renal disease. It should be emphasized that specific gravity measurement is most valid in the first urine sample voided before fluid therapy is initiated because successful fluid therapy will result in production of dilute urine despite continued azotemia. Other disorders that may result in a decreased ability to concentrate urine in the face of dehydration include septicemia or endotoxemia, nephrogenic diabetes insipidus, washout of the medullary interstitium, or pituitary or hypothalamic diseases that lead to central diabetes insipidus. The pH of equine urine is usually alkaline (7.5 to 9.0). High-intensity exercise or bacteriuria can result in acidic pH. The latter can further result in an ammonia odor to the sample because of breakdown of urea by bacteria with urease activity. Production of more dilute urine usually results in a decrease in urine pH toward the neutral value. Commercially available urine reagent strips can yield false-positive results for protein when alkaline samples are tested. The presence of proteinuria is best assessed by performing the semi-quantitative sulfosalicylic acid precipitation test or by specific quantification with a colorimetric assay (as for cerebrospinal fluid) and by comparing the result with urine creatinine concentration in the form of a urine protein-to-creatinine ratio. The use of a cutoff value of 1.0 or less was found in a small group of normal ponies and horses. Proteinuria may occur with pyuria, bacteriuria, or glomerular disease, or transiently following exercise. Transient proteinuria is seen in neonatal foals after ingestion of colostrum. Normal equine urine should not contain glucose. Glucosuria may accompany hyperglycemia associated with the causes described previously or with administration of dextrose-containing fluids or parenteral nutrition products. In addition, glucosuria may accompany sedation with α2-agonists or exogenous corticosteroid administration. When glucosuria is detected in the absence of hyperglycemia, primary tubular dysfunction should be suspected. A positive result for blood on a urine reagent strip can result from the presence of hemoglobin, myoglobin, or intact red blood cells (RBCs) in the urine sample. Evaluation of serum for hemolysis and of urine sediment for RBCs, combined with an ammonium sulfate precipitation test to detect myoglobin, can help to differentiate between these pigments. Urine sediment should be evaluated for cells, casts, and bacteria within 30 to 60 minutes after collection because cells may lyse in hypotonic urine. Fewer than 5 RBCs per high-power field (hpf) can be seen in an atraumatically collected urine sample. Increases in the number of urinary RBCs/hpf can result from inflammation, infection, toxemia, neoplasia, or exercise. Pyuria (>5 white blood cells/hpf) is seen most commonly with infectious or inflammatory disorders. Casts are molds of protein and cells that form in tubular lumens and subsequently pass into the bladder. They are rare in normal equine urine but may be found with inflammatory or infectious processes. Casts are relatively unstable in alkaline urine; evaluation of urine sediment should thus be performed as soon as possible after collection to ensure accurate assessment. Normal equine urine should have few to no bacteria. The absence of bacteria on sediment evaluation does not rule out their presence, however, and bacterial culture of urine collected by catheterization or cystocentesis (in foals) should be performed in suspected cases of pyelonephritis or cystitis. Equine urine is rich in crystals. Most are calcium carbonate crystals of variable size, but triple-phosphate crystals and an occasional calcium oxalate crystal can also be seen in normal equine urine. In some samples, addition of a few drops of a 10% acetic acid solution may be necessary to dissolve crystals for accurate assessment of urine sediment. γ-Glutamyltransferase (GGT) is an enzyme located in the brush border of the epithelial cells lining renal tubules. The presence of GGT activity in urine arises from proximal renal tubular cell turnover, and the activity increases with renal tubular damage and sloughing of epithelium into the tubular lumen. Values for urine GGT activity are expressed as a ratio to urine creatinine (uCr) concentration, as follows, with a value higher than 25 considered abnormal:
Examination of the Urinary System
History and Physical Examination
Hematology and Serum Biochemistry
Urinalysis
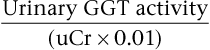
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


